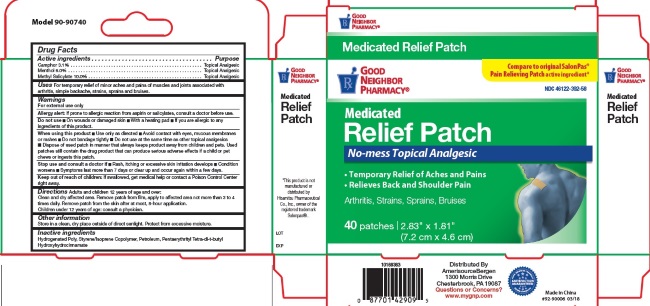
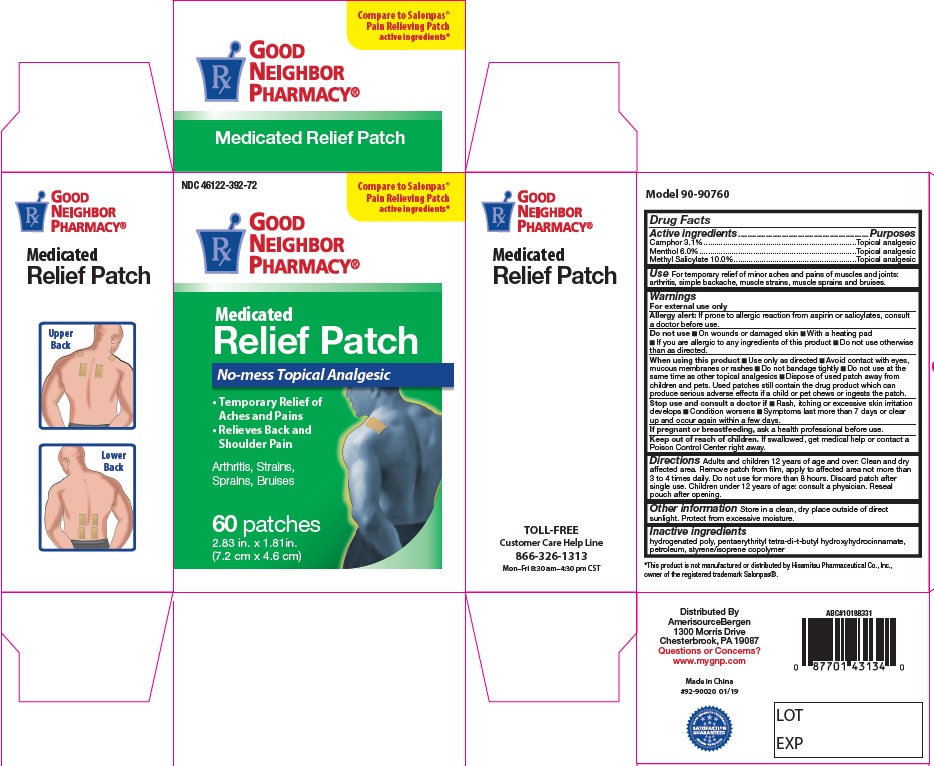
Label: PAIN RELIEF PATCHES- camphor, menthol, methyl salicylate patch
- NDC Code(s): 46122-392-58, 46122-392-60, 46122-392-72
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
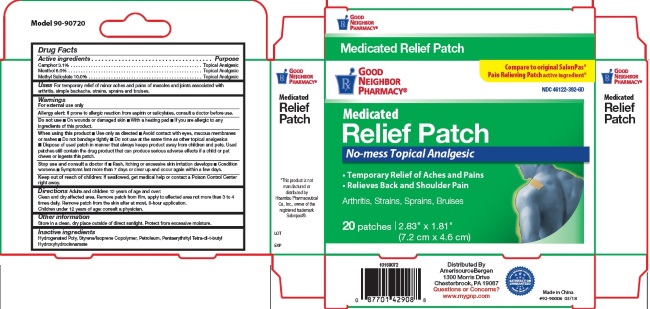
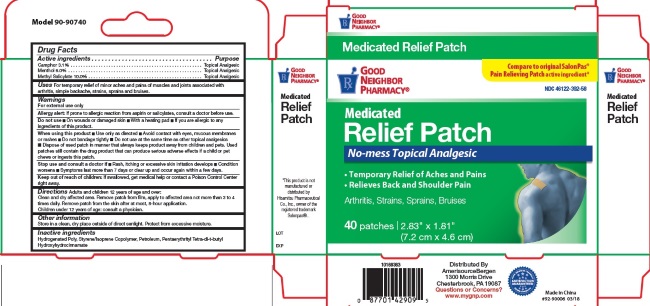
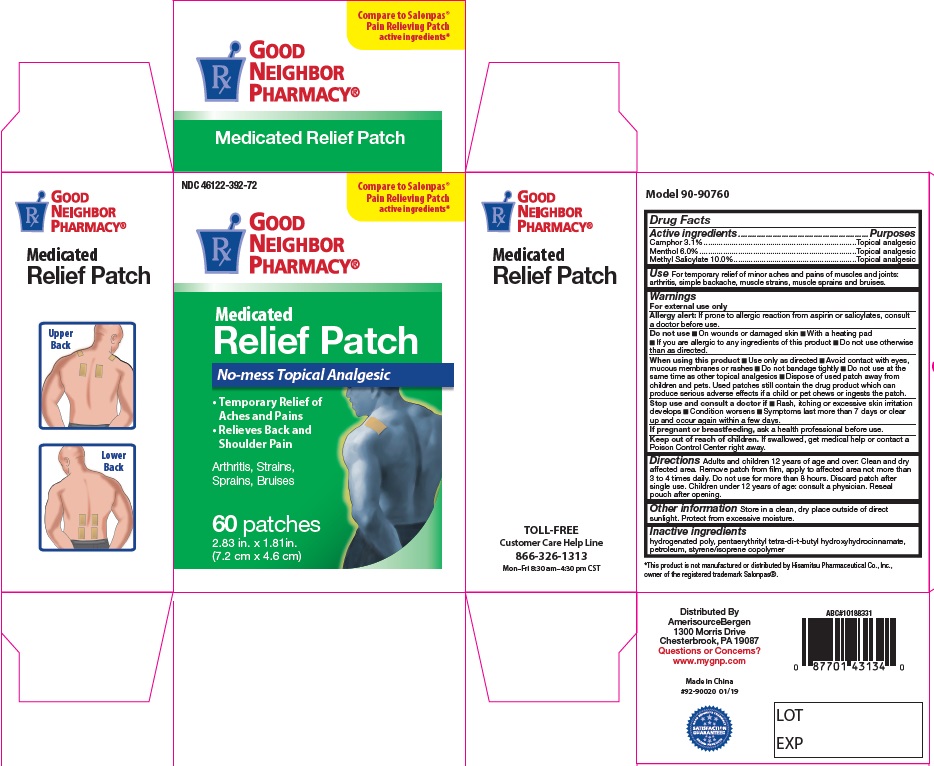
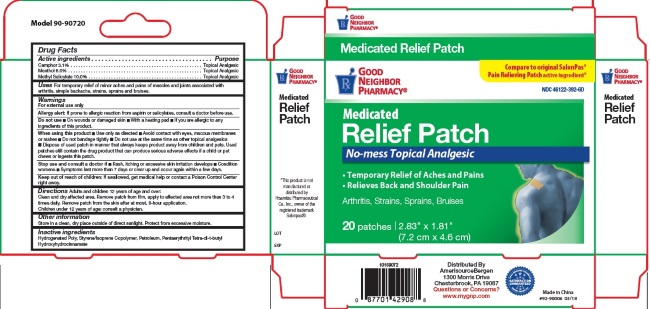
- Active Ingredients
- For External Use Only
- Do not use:
-
When using this product
- Use only as directed
- Avoid contact with eyes, mucous membranes or rashes
- Do not bandage tightly
- Do not use at the same time as other topical analgesics
- Dispose of used patch in manner that keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- Stop use and consult a doctor
- Keep out of reach of children
- Uses
-
Directions
Adults and children 12 years of age and over: Clean and dry affected area. Remove patch from film, apply to affected area not more than 3 to 4 times daily. Remove patch from the skin after at most, 8-hour application.
Children under 12 years of age: consult physician.
- Pain Relief Patch Label
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PATCHES
camphor, menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-392 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.1 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) Product Characteristics Color Score Shape SQUARE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-392-60 20 in 1 BOX 01/01/2018 1 9 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:46122-392-58 40 in 1 BOX 01/01/2018 2 9 g in 1 PATCH; Type 0: Not a Combination Product 3 NDC:46122-392-72 60 in 1 BOX 01/01/2018 3 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2018 Labeler - Amerisource Bergen (007914906) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(46122-392)