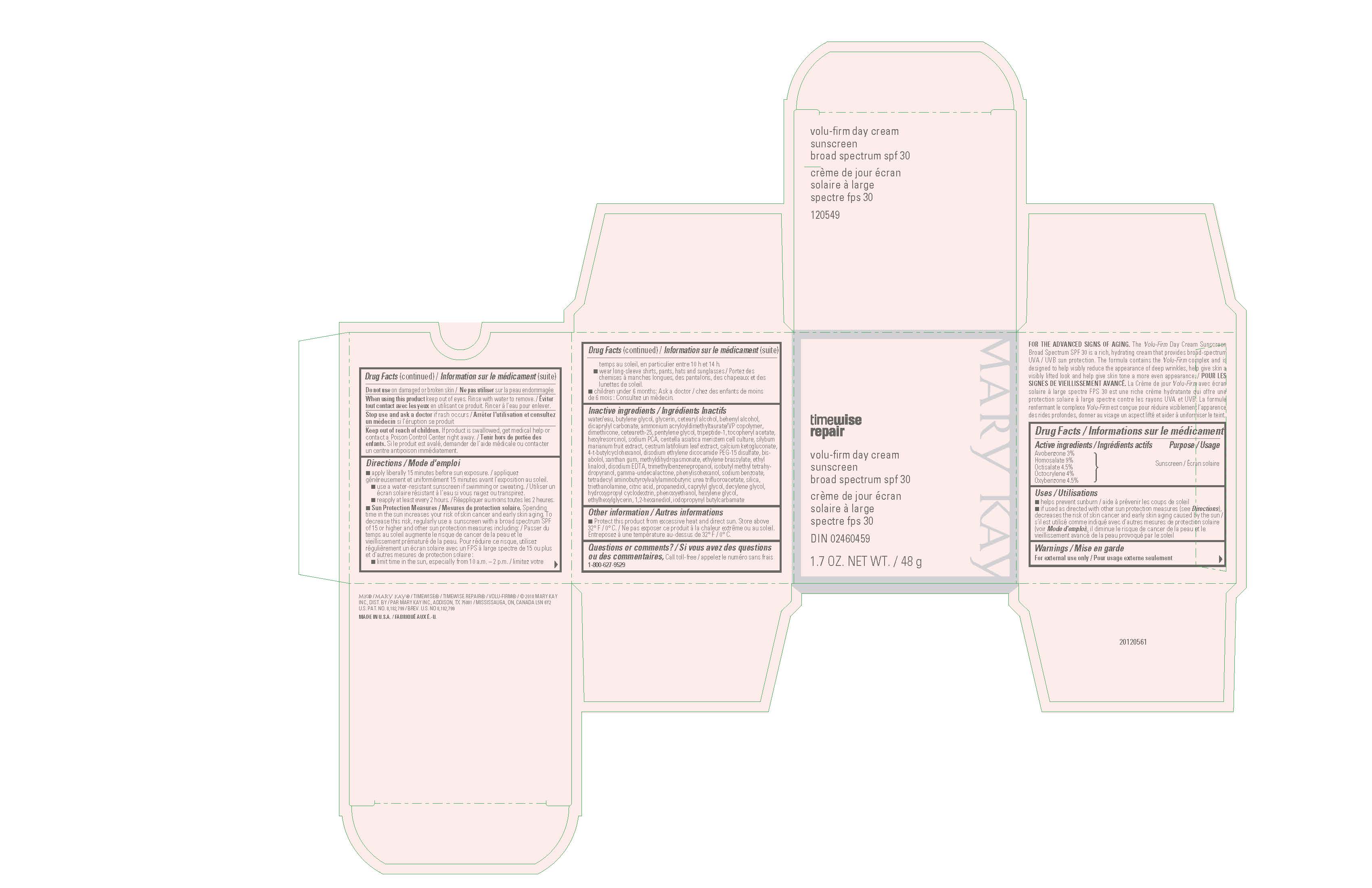
Label: MARY KAY TIMEWISE REPAIR VOLU-FIRM DAY CREAM BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate, octocrylene, oxybenzone cream
- NDC Code(s): 51531-0549-3, 51531-0549-7
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive ingredients
water, butylene glycol, glycerin, cetearyl alcohol, behenyl alcohol, dicaprylyl carbonate, ammonium acryloyldimethyltaurate/VP copolymer, dimethicone, ceteareth-25, pentylene glycol, tripeptide-1, tocopheryl acetate, hexylresorcinol, sodium PCA, centella asiatica meristem cell culture, silybum marianum fruit extract, cestrum latifolium leaf extract, calcium ketogluconate, 4-t-butylcyclohexanol, disodium ethylene dicocamide PEG-15 disulfate, bisabolol, xanthan gum, methyldihydrojasmonate, ethylene brassylate, ethyl linalool, disodium EDTA, trimethylbenzenepropanol, isobutyl methyl tetrahydropyranol, gamma-undecalactone, phenylisohexanol, sodium benzoate, tetradecyl aminobutyroylvalylaminobutyric urea trifuoroacetate, silica, triethanolamine, citric acid, propanediol, caprylyl glycol, decylene glycol, hydroxypropyl cyclodextrin, phenoxyethanol, hexylene glycol, ethylhexylglycerin, 1,2-hexanediol, magnesium chloride, iodopropynyl butylcarbamate
- Other information
- Questions or comments?
- Principal Display Panel - 48 g carton
-
INGREDIENTS AND APPEARANCE
MARY KAY TIMEWISE REPAIR VOLU-FIRM DAY CREAM BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-0549 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DOCOSANOL (UNII: 9G1OE216XY) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) DIMETHICONE (UNII: 92RU3N3Y1O) CETEARETH-25 (UNII: 8FA93U5T67) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) CALCIUM 2-KETOGLUCONATE (UNII: 8K5SM7SI6Y) HEXYLRESORCINOL (UNII: R9QTB5E82N) 4-TERT-BUTYLCYCLOHEXANOL (UNII: K0H1405S9C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYL LINALOOL (UNII: SF2JS9GF5T) TROLAMINE (UNII: 9O3K93S3TK) LEVOMENOL (UNII: 24WE03BX2T) DECYLENE GLYCOL (UNII: S57M60MI88) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROXYPROPYL .ALPHA.-CYCLODEXTRIN (UNII: ZFR0T80O4Y) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CENTELLA ASIATICA (UNII: 7M867G6T1U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) TRIMETHYLBENZENEPROPANOL (UNII: 7S411YY2VY) 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) MILK THISTLE (UNII: U946SH95EE) .GAMMA.-UNDECALACTONE (UNII: QB1T0AG2YL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENYLISOHEXANOL (UNII: M56178H183) PROPANEDIOL (UNII: 5965N8W85T) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) CESTRUM LATIFOLIUM LEAF (UNII: O2618B6E88) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM BENZOATE (UNII: OJ245FE5EU) TETRADECYL AMINOBUTYROYLVALYLAMINOBUTYRIC UREA TRIFLUOROACETATE (UNII: 0UBP26S1LG) PREZATIDE (UNII: 39TG2H631E) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-0549-7 1 in 1 CARTON 05/16/2019 1 48 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:51531-0549-3 9 g in 1 TUBE; Type 0: Not a Combination Product 05/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/16/2019 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-0549) Establishment Name Address ID/FEI Business Operations Englewood Lab Inc. 172198223 manufacture(51531-0549)