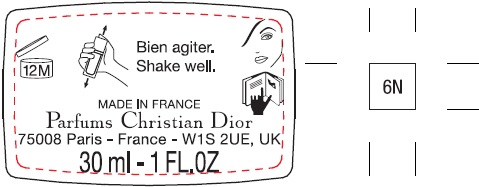
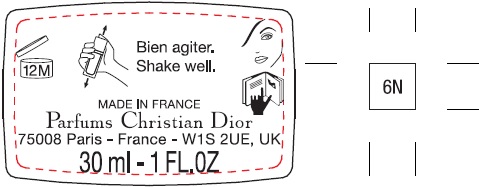
Label: FOREVER SKIN GLOW 24H WEAR RADIANT FOUNDATION PERFECTION AND HYDRATION CONCENTRATED FLORAL SKINCARE WITH SUNCREEN BROAD SPECTRUM SPF 15 6N- octisalate, titanium dioxide emulsion
- NDC Code(s): 61957-3107-0, 61957-3107-1, 61957-3107-2, 61957-3107-7
- Packager: Parfums Christian Dior
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- Children under 6 months of age: ask a doctor.
- Other information
-
Inactive ingredients
AQUA (WATER) • ALCOHOL • METHYL TRIMETHICONE • ISODODECANE • PHENYL TRIMETHICONE • DIMETHICONE • GLYCERIN • PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • TRIMETHYL PENTAPHENYL TRISILOXANE • ACRYLATES/DIMETHICONE COPOLYMER • BUTYLENE GLYCOL • CETYL DIMETHICONE • DISTEARDIMONIUM HECTORITE • CELLULOSE • CETYL PEG/PPG-10/1 DIMETHICONE • SODIUM MYRISTOYL GLUTAMATE • PARFUM (FRAGRANCE) • PROPYLENE CARBONATE • CHLORPHENESIN • VP/VA COPOLYMER • ALUMINUM HYDROXIDE • HIBISCUS SABDARIFFA FLOWER EXTRACT • TROMETHAMINE • TOCOPHEROL • ALUMINA • STEARIC ACID • TROPAEOLUM MAJUS FLOWER/LEAF/STEM EXTRACT • HEXADECENE • HYDROLYZED VIOLA TRICOLOR EXTRACT • 1,2-HEXANEDIOL • CAPRYLYL GLYCOL • IRIS FLORENTINA ROOT EXTRACT • SODIUM BENZOATE • POTASSIUM SORBATE • PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE. [MAY CONTAIN: CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77891 (TITANIUM DIOXIDE)].
N° 16030/Z - Package Labeling:30ml
- Package Labeling:20ml
- Package Labeling: 0.7ml
- Package Labeling: 2.7ml
-
INGREDIENTS AND APPEARANCE
FOREVER SKIN GLOW 24H WEAR RADIANT FOUNDATION PERFECTION AND HYDRATION CONCENTRATED FLORAL SKINCARE WITH SUNCREEN BROAD SPECTRUM SPF 15 6N
octisalate, titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61957-3107 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) TRIMETHYL PENTAPHENYL TRISILOXANE (UNII: HE7ABK56N5) 2-ETHYLHEXYL ACRYLATE, METHACRYLATE, METHYL METHACRYLATE, OR BUTYL METHACRYLATE/HYDROXYPROPYL DIMETHICONE COPOLYMER (30000-300000 MW) (UNII: S7ZA3CCJ4M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POWDERED CELLULOSE (UNII: SMD1X3XO9M) SODIUM MYRISTOYL GLUTAMATE (UNII: AYU7QD893W) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CHLORPHENESIN (UNII: I670DAL4SZ) COPOVIDONE K25-31 (UNII: D9C330MD8B) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) TROMETHAMINE (UNII: 023C2WHX2V) TOCOPHEROL (UNII: R0ZB2556P8) ALUMINUM OXIDE (UNII: LMI26O6933) STEARIC ACID (UNII: 4ELV7Z65AP) TROPAEOLUM MAJUS FLOWERING TOP (UNII: RGT30824HY) HEXADECENE (MIXED ISOMERS) (UNII: 38H8547VP0) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) IRIS X GERMANICA NOTHOVAR. FLORENTINA ROOT (UNII: M30XO5X4XD) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61957-3107-0 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2022 2 NDC:61957-3107-1 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2022 3 NDC:61957-3107-7 0.7 mL in 1 PACKET; Type 0: Not a Combination Product 01/01/2024 4 NDC:61957-3107-2 2.7 mL in 1 TUBE; Type 0: Not a Combination Product 01/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2022 Labeler - Parfums Christian Dior (275252245)