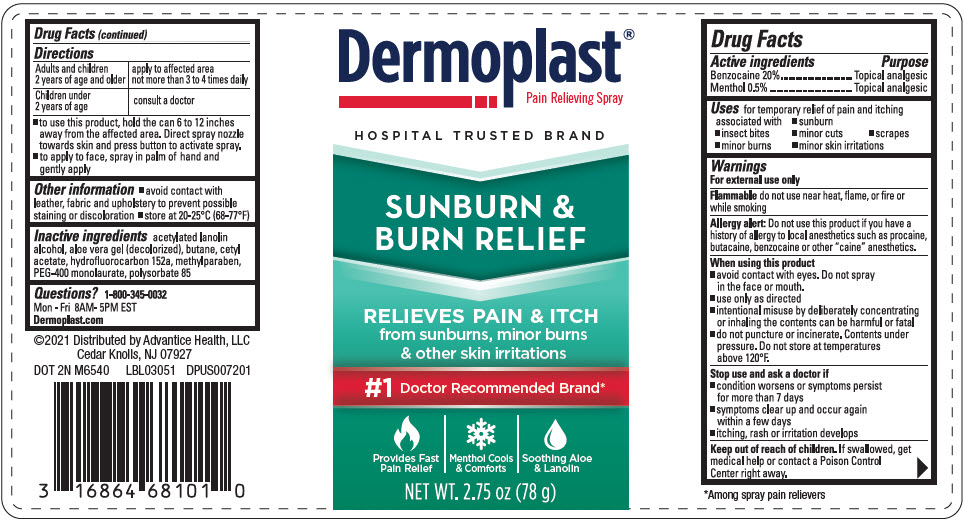
Label: DERMOPLAST SUNBURN AND BURN RELIEF- benzocaine and levomenthol spray
- NDC Code(s): 16864-681-01
- Packager: Advantice Health, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
Flammabledo not use near heat, flame, or fire or while smoking
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product
- avoid contact with eyes. Do not spray in the face or mouth.
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents can be harmful or fatal
- do not puncture or incinerate. Contents under pressure. Do not store at temperatures above 120°F.
-
Directions
Adults and children 2 years of age and older apply to affected area not more than 3 to 4 times daily Children under 2 years of age consult a doctor - to use this product, hold the can 6 to 12 inches away from the affected area. Direct spray nozzle towards skin and press button to activate spray.
- to apply to face, spray in palm of hand and gently apply
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 78 g Can Label
-
INGREDIENTS AND APPEARANCE
DERMOPLAST SUNBURN AND BURN RELIEF
benzocaine and levomenthol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16864-681 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PEG-8 LAURATE (UNII: 762O8IWA10) POLYSORBATE 85 (UNII: A7F3N56197) METHYLPARABEN (UNII: A2I8C7HI9T) ALOE VERA LEAF (UNII: ZY81Z83H0X) ACETYLATED LANOLIN ALCOHOLS (UNII: SNN716810P) CETYL ACETATE (UNII: 4Q43814HXS) BUTANE (UNII: 6LV4FOR43R) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16864-681-01 78 g in 1 CAN; Type 0: Not a Combination Product 05/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/15/2021 04/30/2024 Labeler - Advantice Health, LLC (192527062)