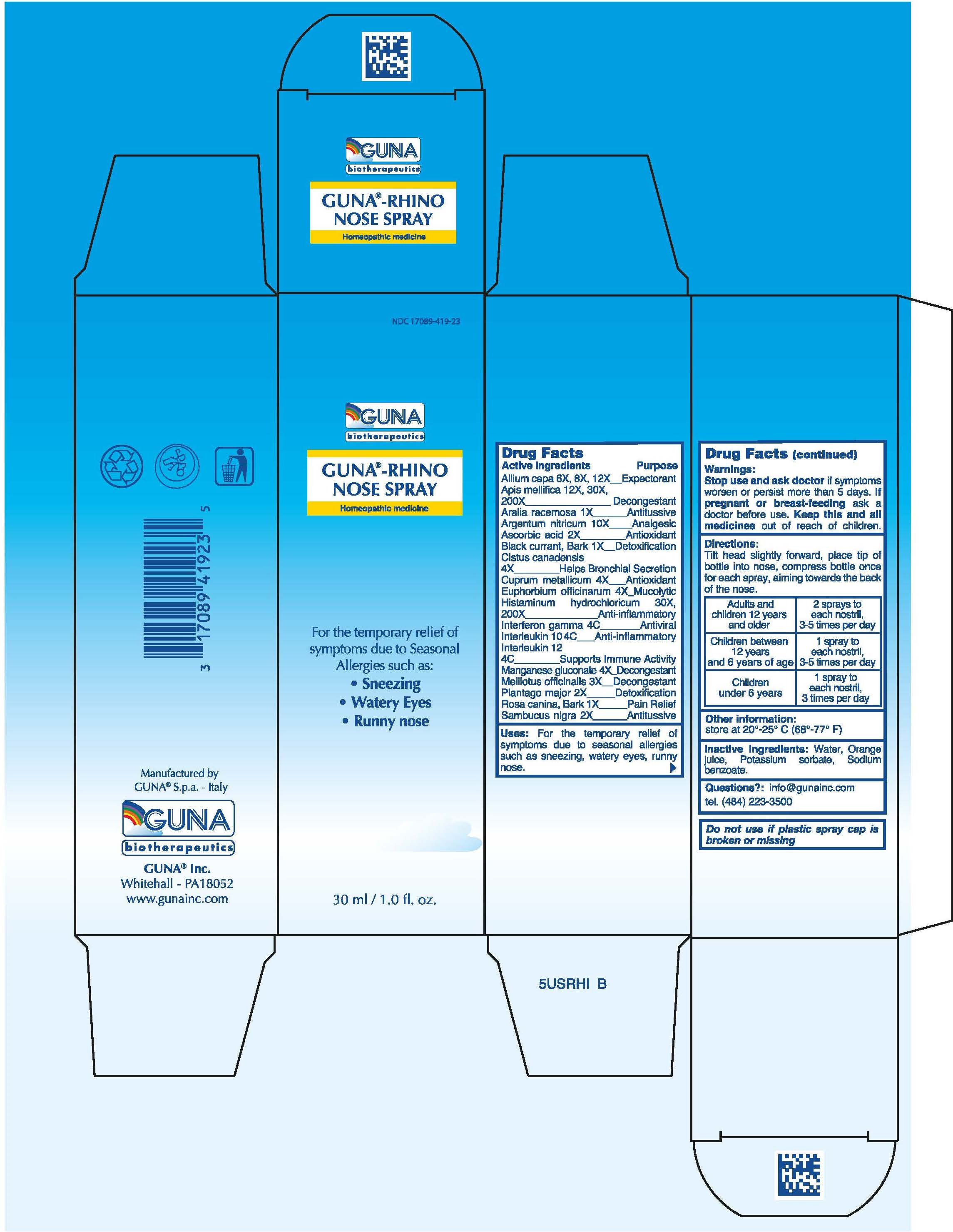
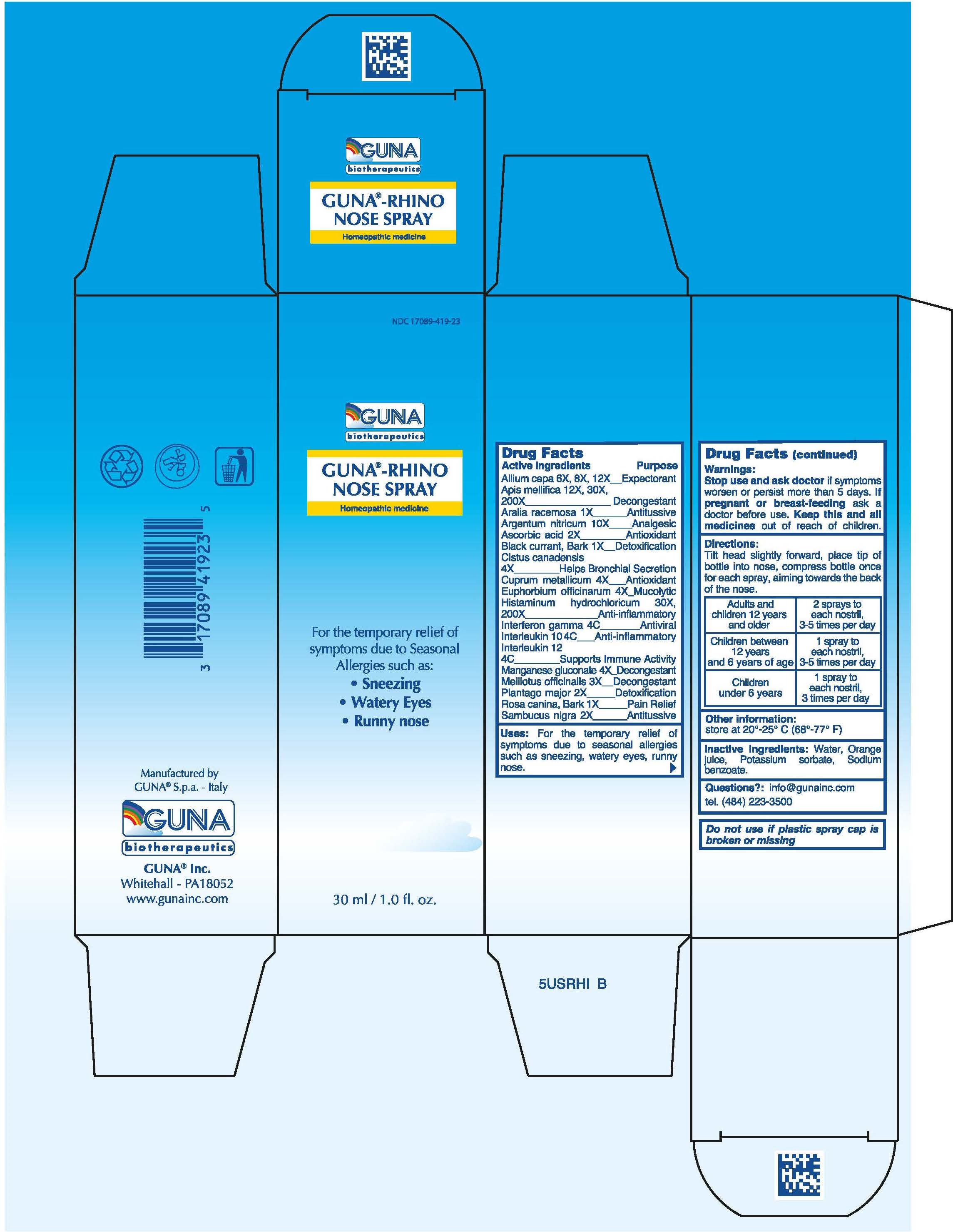
Label: GUNA-RHINO NOSE (apis mellifera - aralia racemosa root - ascorbic acid - black currant - copper - euphorbia resinifera r...view full title
- NDC Code(s): 17089-419-23
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSEALIUM CEPA 6X, 8X, 12X EXPECTORANT - APIS MELLIFICA 12x, 30X, 200X DECONGESTANT - ARALIA RACEMOSA 1X ANTITUSSIVE ...
-
USESFor the temporary relief of symptoms due to Seasonal Allergies such as: Sneezing, Watery Eyes, Runny nose
-
WARNINGSStop use and ask doctor if symptoms worsen or persist more than 5 days
-
PREGNANCYIf pregnant or breast-feeding ask a doctor before use
-
WARNINGSKeep this and all medicines out of reach of children
-
DIRECTIONSAdults and children 12 years and older 2 sprays to each nostril , 3-5 times per day - Children between 12 years and 6 years of age 1 spray to each nostril, 3-5 times per day ...
-
QUESTIONSQuestions?: info@gunainc.com - Tel. (484) 223-3500
-
INDICATIONS & USAGETilt head slightly forward, place tip of bottle into nose, compress bottle once for each spray, aiming towards the back of the nose.
-
INACTIVE INGREDIENTInactive ingredients: Water, Orange juice, Potassium sorbate, Sodium benzoate
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information