Label: TRIAMTERENE AND HYDROCHLOROTHIAZIDE tablet
- NDC Code(s): 71335-0326-1, 71335-0326-2, 71335-0326-3, 71335-0326-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 60505-2657
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
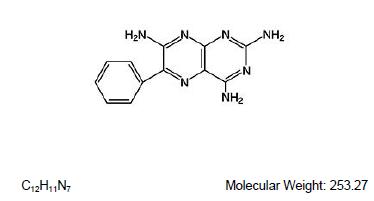
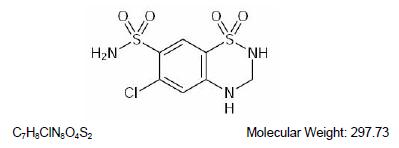
DESCRIPTIONTriamterene and hydrochlorothiazide combines triamterene, a potassium-conserving diuretic, with the natriuretic agent, hydrochlorothiazide. Each Triamterene and Hydrochlorothiazide 37.5 mg/25 mg ...
-
CLINICAL PHARMACOLOGYTriamterene and hydrochlorothiazide is a diuretic, antihypertensive drug product, principally due to its hydrochlorothiazide component; the triamterene component reduces the excessive potassium ...
-
INDICATIONS AND USAGEThis fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked. Triamterene and ...
-
CONTRAINDICATIONSHyperkalemia - Triamterene and hydrochlorothiazide should not be used in the presence of elevated serum potassium levels (greater than or equal to 5.5 mEq/liter). If hyperkalemia develops ...
-
WARNINGSHyperkalemia - Abnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-conserving diuretic combinations, including triamterene and ...
-
PRECAUTIONSGeneral - Electrolyte Imbalance and BUN Increases - Patients receiving triamterene and hydrochlorothiazide should be carefully monitored for fluid or electrolyte imbalances, i.e. ...
-
ADVERSE REACTIONSSide effects observed in association with the use of triamterene and hydrochlorothiazide tablets, other combination products containing triamterene/hydrochlorothiazide, and products containing ...
-
OVERDOSAGENo specific data are available regarding triamterene and hydrochlorothiazide overdosage in humans and no specific antidote is available. Fluid and electrolyte imbalances are the most important ...
-
DOSAGE AND ADMINISTRATIONNote: 37.5 mg/25 mg = 37.5 mg triamterene and 25 mg hydrochlorothiazide 75 mg/50 mg = 75 mg triamterene and 50 mg hydrochlorothiazide - The usual dose of Triamterene and ...
-
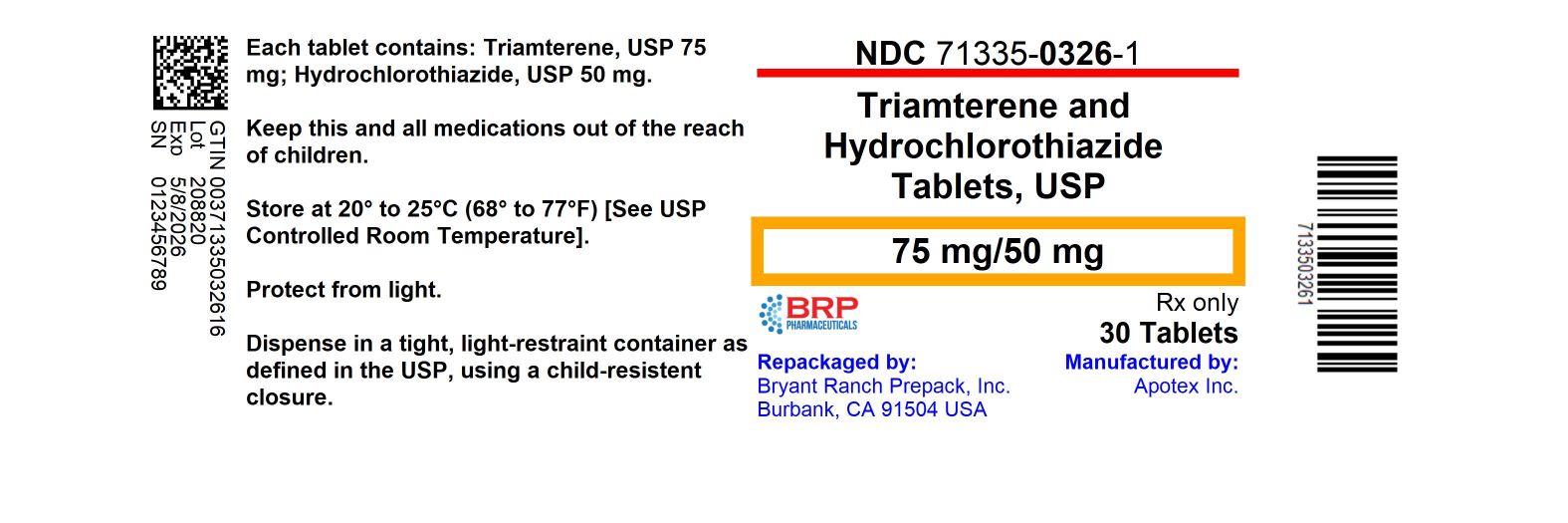
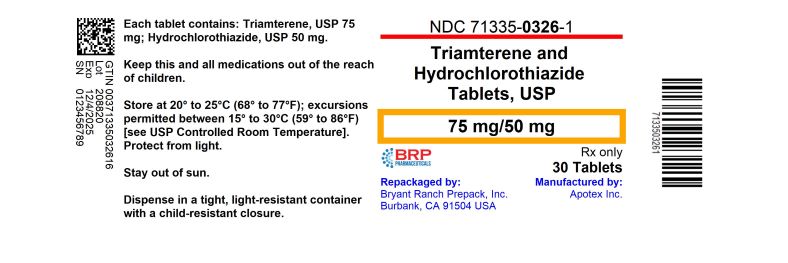
HOW SUPPLIEDTriamterene and Hydrochlorothiazide Tablets, USP, 75 mg/50 mg are available for oral administration as yellow, oval biconvex tablets, scored and engraved “75” bisect “50” on one side, “APO” on the ...
-
PRINCIPAL DISPLAY PANELTriamterene and Hydrochlorothiazide Tablets 75 mg/50 mg
-
INGREDIENTS AND APPEARANCEProduct Information