Label: PVP-IODINE SWABSTICK THREES- povidone-iodine swab
- NDC Code(s): 65517-0033-1, 65517-0033-3
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient:
- Purpose:
- Use:
-
Warnings:
For external use only.
- Do not apply to persons allergic to iodine. Do not use in the eyes.
- Directions:
- Other Information:
- Inactive Ingredients:
-
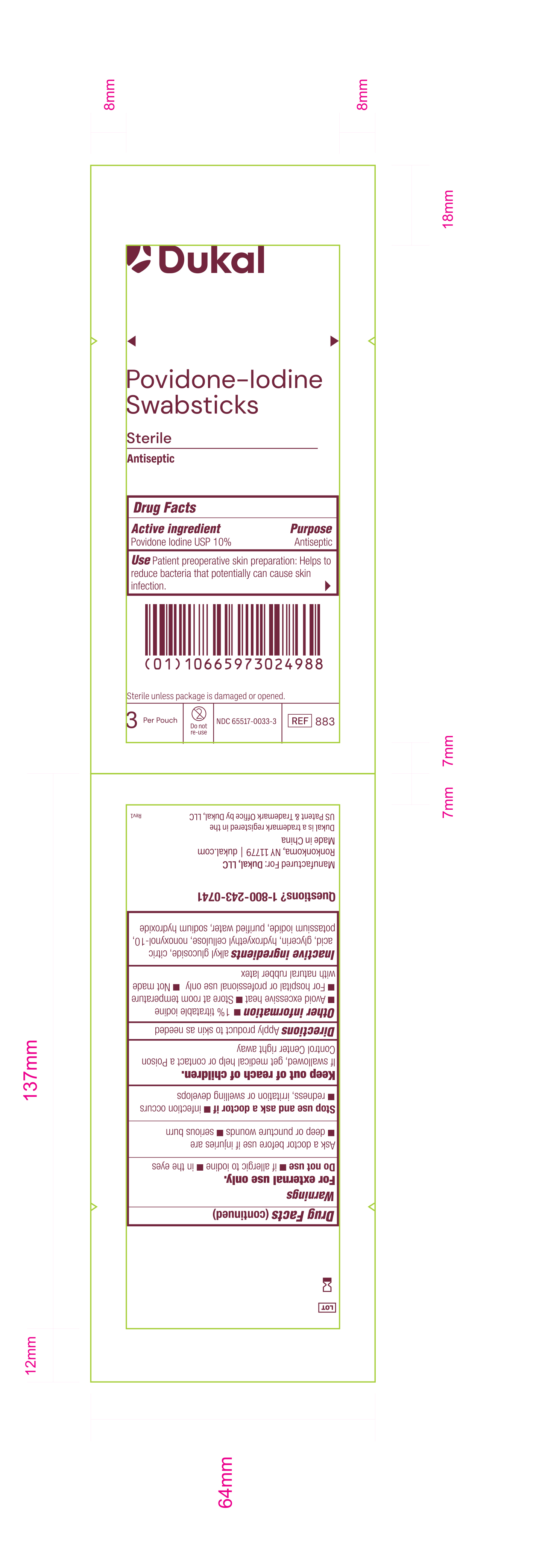
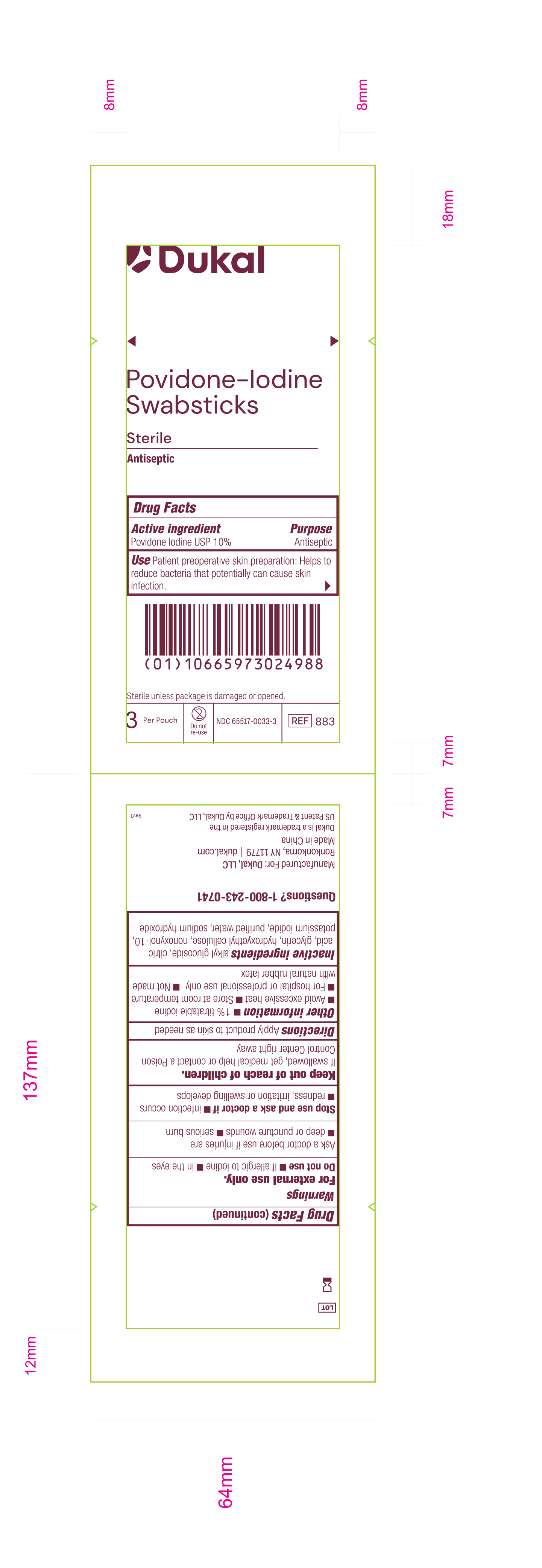
Principal Display Panel - PVP-I Prep Swabstick Sterile 883 Pouch Label
Dukal
Povidone-IodineSwabstick
Sterile
Antiseptic
Drug Facts
Active Ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin reparation: Helps to reduce bacteria that potentially can cause skin infection.
Sterile unless package is damaged or opened.
3 Per Pouch - Do not re-use - NDC 6551-0033-3 - REF 883
-
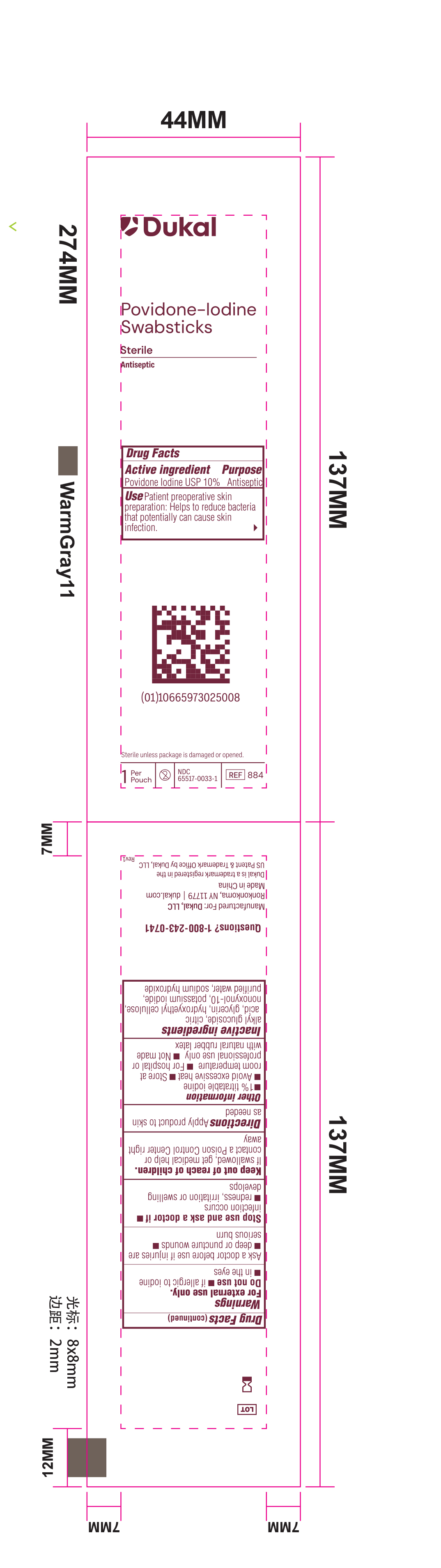
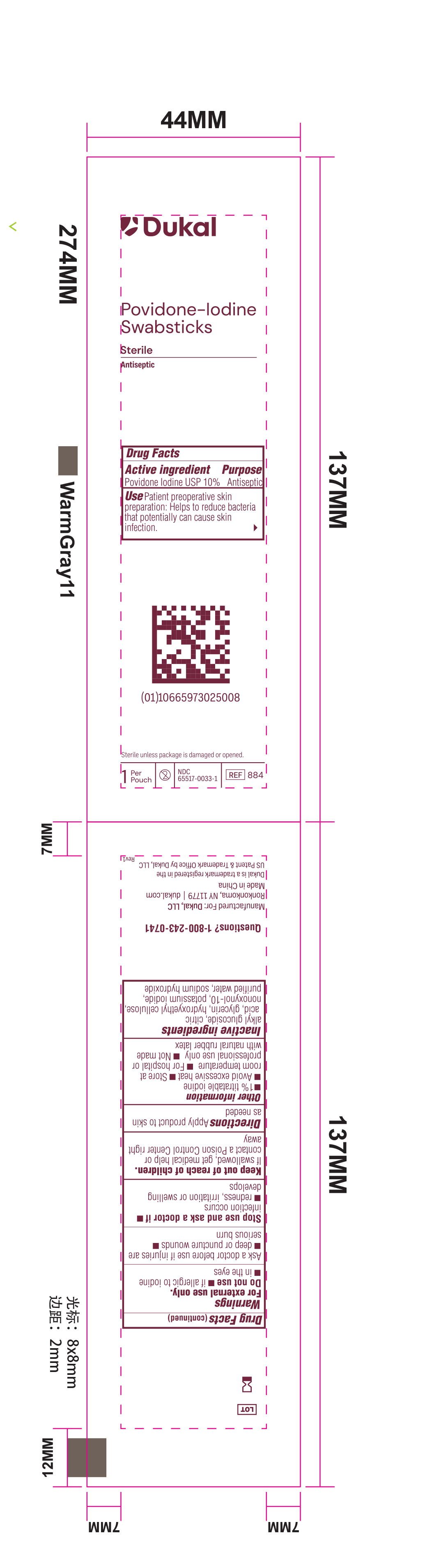
Principal Display Panel - PVP-I Prep Swabstick Sterile 884 Pouch Label
Dukal
Povidone-IodineSwabstick
Sterile
Antiseptic
Drug Facts
Active Ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin reparation: Helps to reduce bacteria that potentially can cause skin infection.
Sterile unless package is damaged or opened.
1 Per Pouch - 2 - NDC 6551-0033-1 - REF 884
-
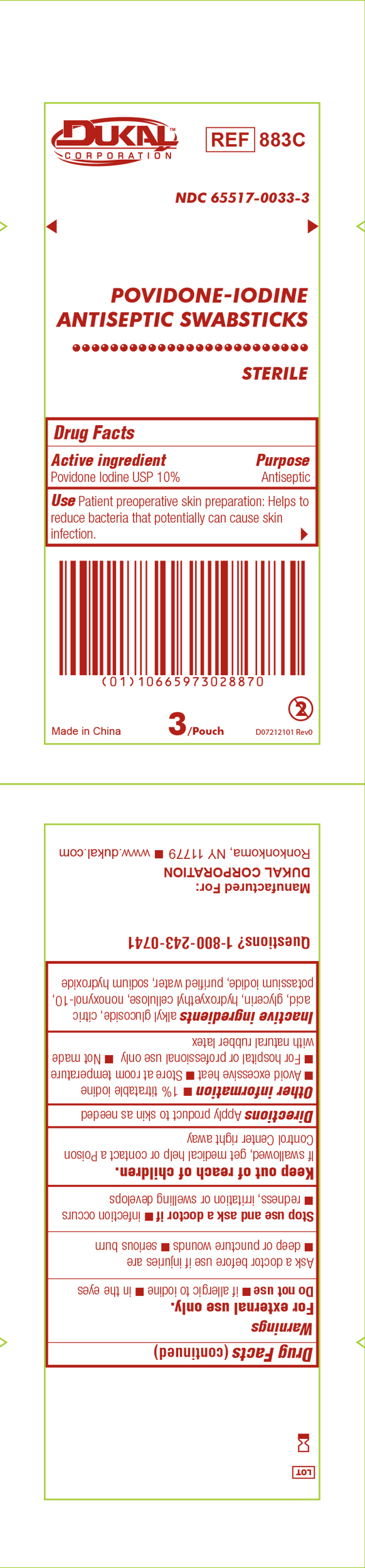
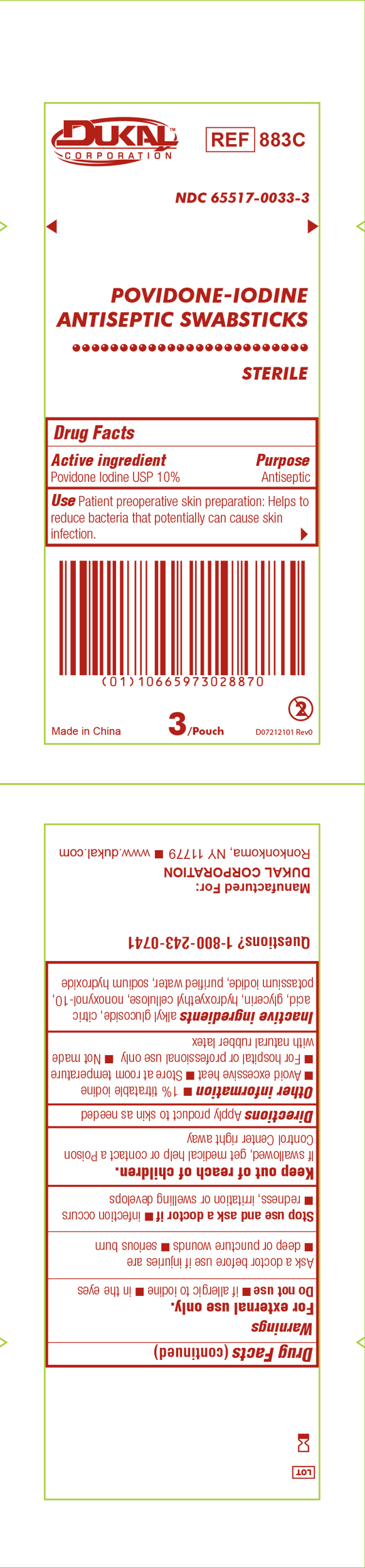
Principal Display Panel - PVP-I Prep Swabstick Sterile 883C Pouch Label
Dukal
Povidone-IodineSwabstick
Sterile
Antiseptic
Drug Facts
Active Ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin reparation: Helps to reduce bacteria that potentially can cause skin infection.
Sterile unless package is damaged or opened.
3 Per Pouch - Do not re-use - NDC 6551-0033-3 - REF 883C
-
INGREDIENTS AND APPEARANCE
PVP-IODINE SWABSTICK THREES
povidone-iodine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-0033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) NONOXYNOL-10 (UNII: K7O76887AP) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) POTASSIUM IODIDE (UNII: 1C4QK22F9J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0033-1 1000 in 1 CASE 01/31/2018 1 1 in 1 POUCH 1 2.3 mL in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:65517-0033-3 500 in 1 CASE 01/31/2018 2 3 in 1 POUCH 2 1.8 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/31/2018 Labeler - Dukal LLC (791014871)