Label: ANTISEPTIC SINOFRESH KOOL BLAST THROAT- mentha piperita spray
- NDC Code(s): 59228-105-11
- Packager: EMS Contract Packaging
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
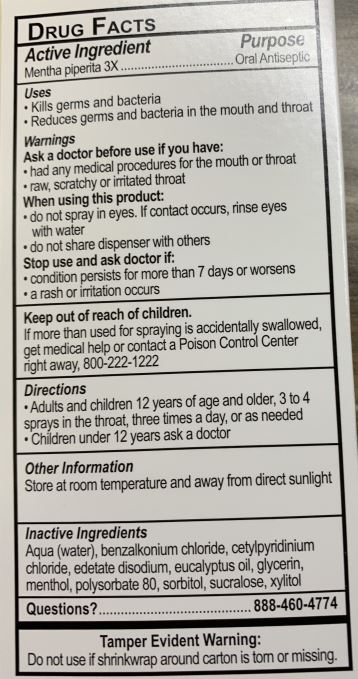
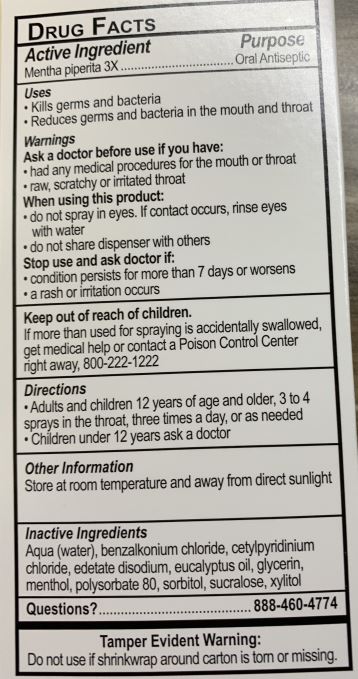
ACTIVE INGREDIENTSMENTHA PIPERITA 3X
-
PURPOSEORAL ANTISEPTIC
-
USESKILLS GERMS AND BACTERIA - REDUCES GERMS AND BACTERIA IN THE MOUTH AND THROAT
-
WARNINGSASK A DOCTOR BEFORE USE IF YOU HAVE: HAD ANY MEDICAL PROCEDURES FOR THE MOUTH OR THROAT - RAW, SCRATCHY, OR IRRITATED THROAT - wHEN USING THIS PRODUCT: DO NOT SPRAY IN EYES. IF CONTACT OCCURS ...
-
KEEP OUT OF REACH OF CHILDRENKEEP OUT OF REACH OF CHILDREN. IF MORE THAN IS USED FOR SPRAYING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY, 800-222-1222
-
DIRECTIONSADULTS AND CHILDREN 12 YEARS AND OLDER, 3 TO 4 SPRAYS IN THE THROAT, THREE TIMES A DAY, OR AS NEEDED - CHILDREN UNDER 12 YEARS ASK A DOCTOR
-
OTHER INFORMATIONSTORE AT ROOM TEMPERATURE, AWAY FROM DIRECT SUNLIGHT
-
INACTIVE INGREDIENTSAQUA (WATER), BENZALKONIUM CHLORIDE, CETYLPYRIDINIUM CHLORIDE, EDETATE DISODIUM, EUCALYPTUS OIL, GLYCERIN, MENTHOL, POLYSORBATE 80, SORBITOL, SUCRALOSE, XYLITOL
-
QUESTIONS?888-460-4774
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information