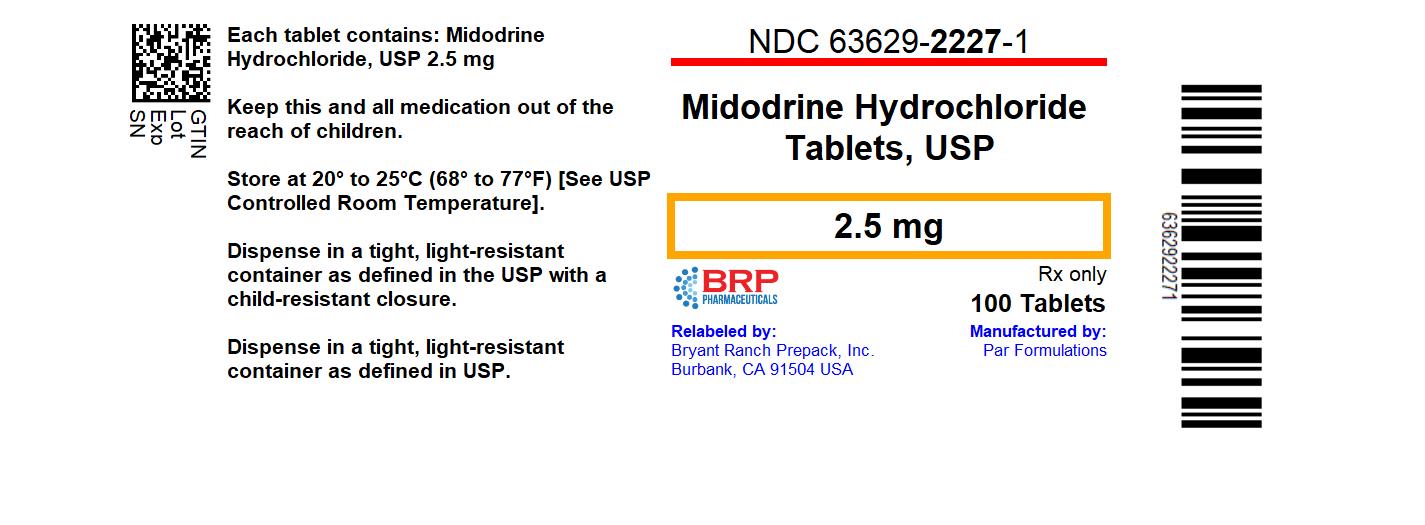
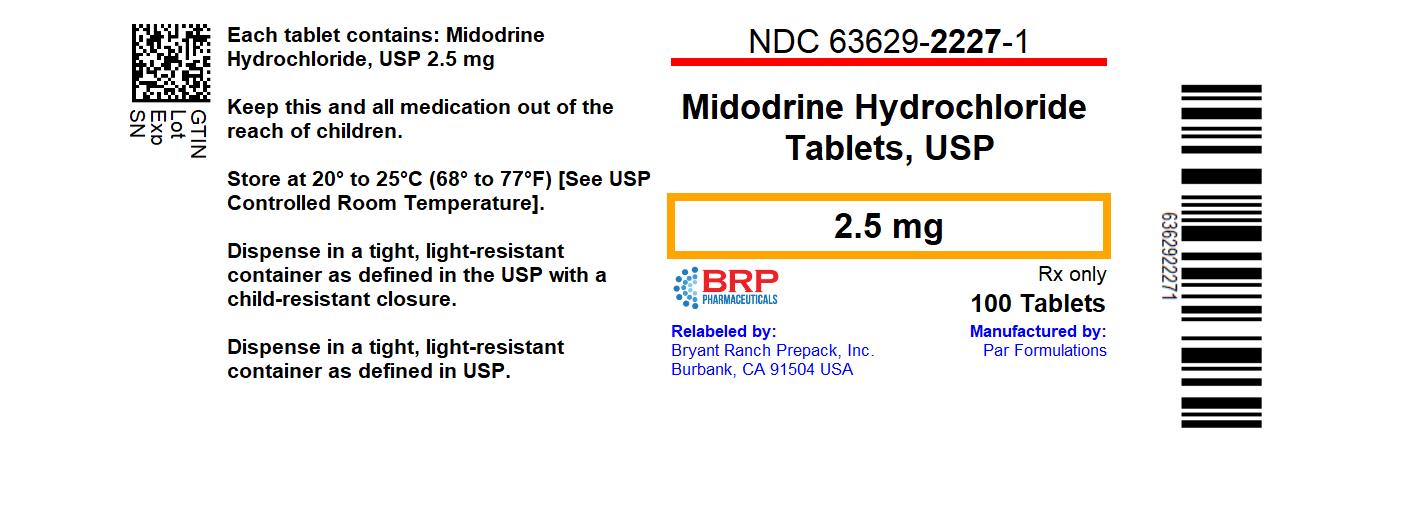
Label: MIDODRINE HYDROCHLORIDE tablet
- NDC Code(s): 63629-2227-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 49884-814
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
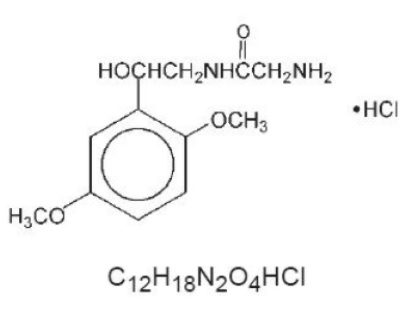
DESCRIPTIONMidodrine hydrochloride is a vasopressor/antihypotensive. The chemical name for midodrine hydrochloride is acetamide, 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]-, monohydrochloride, (±)- ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Midodrine hydrochloride forms an active metabolite, desglymidodrine, that is an alpha1-agonist, and exerts its actions via activation of the alpha-adrenergic receptors of ...
-
Clinical StudiesMidodrine has been studied in 3 principal controlled trials, one of 3-weeks duration and 2 of 1 to 2 days duration. All studies were randomized, double-blind and parallel-design trials in patients ...
-
INDICATIONS AND USAGEMidodrine hydrochloride tablets, USP are indicated for the treatment of symptomatic orthostatic hypotension (OH). Because midodrine hydrochloride tablets, USP can cause marked elevation of supine ...
-
CONTRAINDICATIONSMidodrine hydrochloride tablets are contraindicated in patients with severe organic heart disease, acute renal disease, urinary retention, pheochromocytoma or thyrotoxicosis. Midodrine ...
-
WARNINGSSupine Hypertension: The most potentially serious adverse reaction associated with midodrinehydrochloride therapy is marked elevation of supine arterial blood pressure (supine hypertension) ...
-
PRECAUTIONSGeneral - The potential for supine and sitting hypertension should be evaluated at the beginning of midodrine hydrochloride therapy. Supine hypertension can often be controlled by preventing the ...
-
ADVERSE REACTIONSThe most frequent adverse reactions seen in controlled trials were supine and sitting hypertension; paresthesia and pruritus, mainly of the scalp; goosebumps; chills; urinary urge; urinary ...
-
OVERDOSAGESymptoms of overdose could include hypertension, piloerection (goosebumps), a sensation of coldness and urinary retention. There are 2 reported cases of overdosage with midodrine hydrochloride ...
-
DOSAGE AND ADMINISTRATIONThe recommended dose of midodrine hydrochloride tablets is 10 mg, 3 times daily. Dosing should take place during the daytime hours when the patient needs to be upright, pursuing the activities of ...
-
HOW SUPPLIEDMidodrine Hydrochloride Tablets, USP are available for oral administration, containing 2.5 mg of midodrine hydrochloride, USP. The 2.5 mg tablets are white to off-white, uncoated round shaped ...
-
PRINCIPAL DISPLAY PANELMidodrine Hcl 2.5 mg Tablet, #100
-
INGREDIENTS AND APPEARANCEProduct Information