Label: PAIN RELIEF EXTRA STRENGTH- acetaminophen tablet
- NDC Code(s): 59726-494-05, 59726-494-10, 59726-494-24, 59726-494-50
- Packager: P & L Development, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
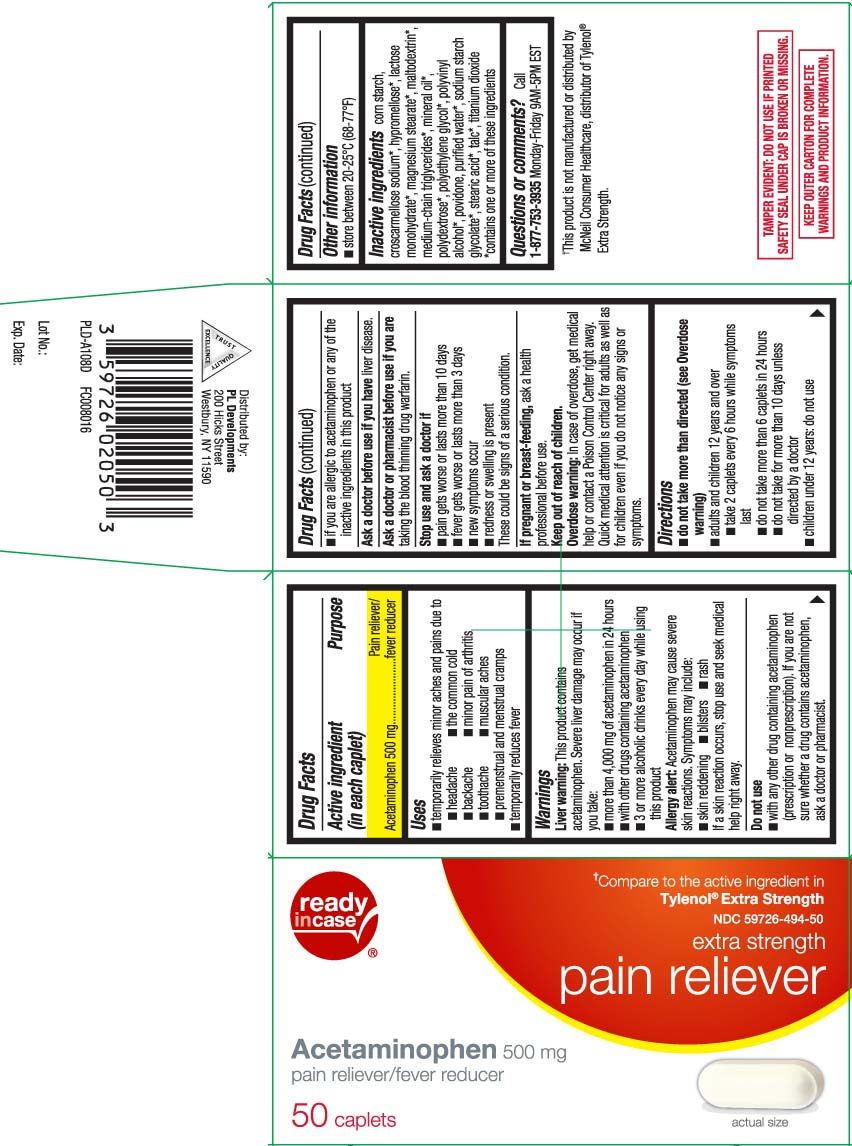
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks ever day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek a medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- Directions
- Other information
-
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, lactose monohydrate*, magnesium stearate*,maltodextrin*, medium-chain triglycerides*, mineral oil*, polydextrose*, polyethylene glycol*, polyvinyl alcohol*, povidone, purified water*, sodium starch glycolate, stearic acid*, talc*, titanium dioxide
*contains one or more of these ingredients
- Questions or comments?
-
Principal Display Panel
Compare to the active ingredient in Tylenol® Extra Strength†
Extra Strength
Pain Reliever
Acetaminophen, 500 mg
Pain Reliever/Fever Reducer
Caplets
†This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Tylenol® Extra Strength.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
Distributed by:
PL Developments
200 Hicks Street
Westbury, NY 11590
- Product Label
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF EXTRA STRENGTH
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59726-494 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) WATER (UNII: 059QF0KO0R) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) MALTODEXTRIN (UNII: 7CVR7L4A2D) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYDEXTROSE (UNII: VH2XOU12IE) Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code TCL341;AV;0821;P500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59726-494-24 1 in 1 BOX 03/31/2016 1 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:59726-494-05 1 in 1 BOX 03/31/2016 2 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:59726-494-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/31/2016 4 NDC:59726-494-50 1 in 1 BOX 03/31/2016 4 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 03/31/2016 Labeler - P & L Development, LLC (800014821)