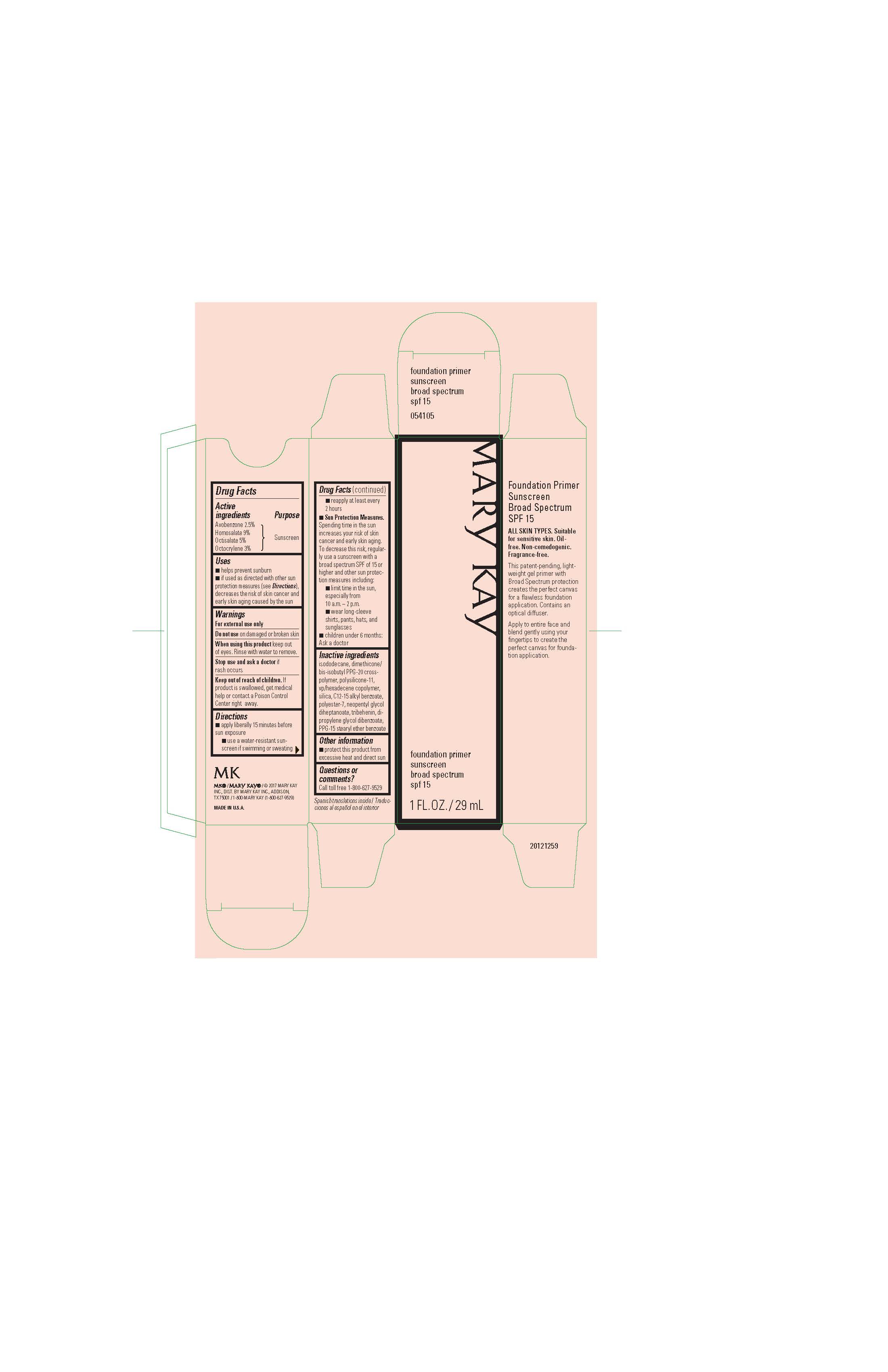
Label: MARY KAY FOUNDATION PRIMER- avobenzone, homosalate, octisalate, octocrylene gel
- NDC Code(s): 51531-0850-0, 51531-0850-1
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
- Inactive ingredients
- Other information
- Questions or comments?
- Principal Display Panel - 29 mL carton
-
INGREDIENTS AND APPEARANCE
MARY KAY FOUNDATION PRIMER
avobenzone, homosalate, octisalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-0850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYESTER-7 (UNII: 0841698D2F) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) TRIBEHENIN (UNII: 8OC9U7TQZ0) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-0850-1 1 in 1 CARTON 03/01/2012 1 29 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:51531-0850-0 0.5 mL in 1 PACKET; Type 0: Not a Combination Product 03/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2012 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-0850)