Label: METOPROLOL SUCCINATE tablet, film coated, extended release
- NDC Code(s): 51079-169-01, 51079-169-20, 51079-170-01, 51079-170-20, view more
- Packager: Mylan Institutional Inc.
- This is a repackaged label.
- Source NDC Code(s): 0378-4595, 0378-4596, 0378-4597
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOPROLOL SUCCINATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METOPROLOL SUCCINATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Metoprolol succinate extended-release tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - Adults - The usual initial dosage is 25 to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved ...
-
3 DOSAGE FORMS AND STRENGTHSMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190 mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of ...
-
4 CONTRAINDICATIONSMetoprolol succinate extended-release tablets are contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, decompensated heart failure, sick sinus syndrome ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: Worsening angina or myocardial infarction - [see - Warnings and Precautions (5)]. Worsening heart failure ...
-
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Untreated hypertension and heart failure during pregnancy can lead to adverse outcomes for the mother and the fetus - (see - Clinical Considerations) ...
-
10 OVERDOSAGESigns and Symptoms:Overdosage of metoprolol succinate extended-release tablets may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include ...
-
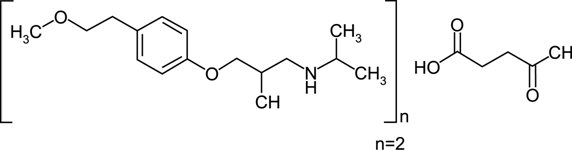
11 DESCRIPTIONMetoprolol succinate is a beta - 1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate extended-release ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoprolol is a beta - 1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...
-
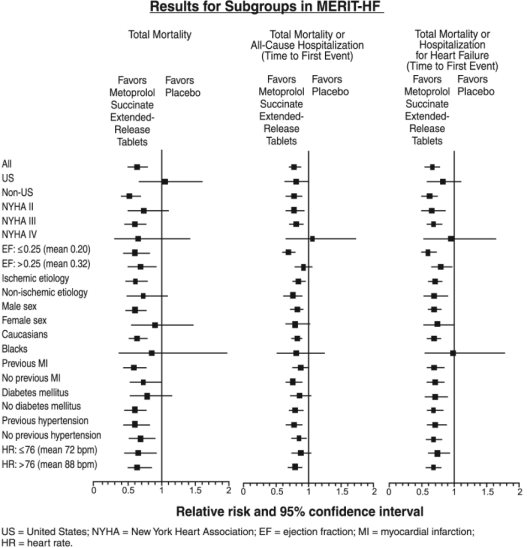
14 CLINICAL STUDIES14.1 Hypertension - In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily metoprolol succinate extended-release tablets (25, 100, or 400 mg) ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg or 95 mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg or 100 mg of metoprolol tartrate ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to take metoprolol succinate extended-release tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient ...
-
PRINCIPAL DISPLAY PANEL - 25 mgNDC 51079-169-20 - Metoprolol - Succinate - Extended-Release - Tablets, USP - 25 mg* CAUTION - Verify Product Dispensed - 100 Tablets (10 x 10) *Each film-coated tablet contains - 23.75 mg ...
-
PRINCIPAL DISPLAY PANEL - 50 mgNDC 51079-170-20 - Metoprolol - Succinate - Extended-Release - Tablets, USP - 50 mg* CAUTION - Verify Product Dispensed - 100 Tablets (10 x 10) *Each film-coated tablet contains - 47.5 mg ...
-
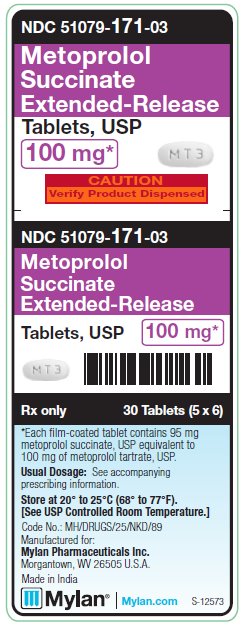
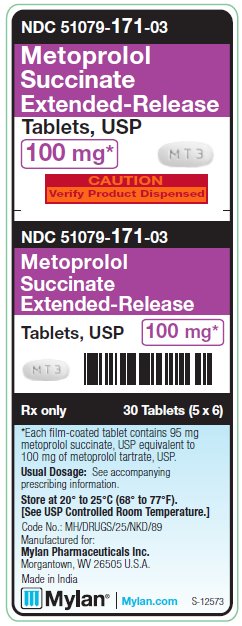
PRINCIPAL DISPLAY PANEL - 100 mgNDC 51079-171-03 - Metoprolol - Succinate - Extended-Release - Tablets, USP - 100 mg* CAUTION - Verify Product Dispensed - 30 Tablets (5 x 6) *Each film-coated tablet contains - 95 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information