Label: VEGETABLE LAXATIVE- sennosides tablet
- NDC Code(s): 68210-5017-4
- Packager: Spirit Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- puropse
- Uses
- Warnings
-
Directions
- take preferably at bedtime or as directed by a doctor
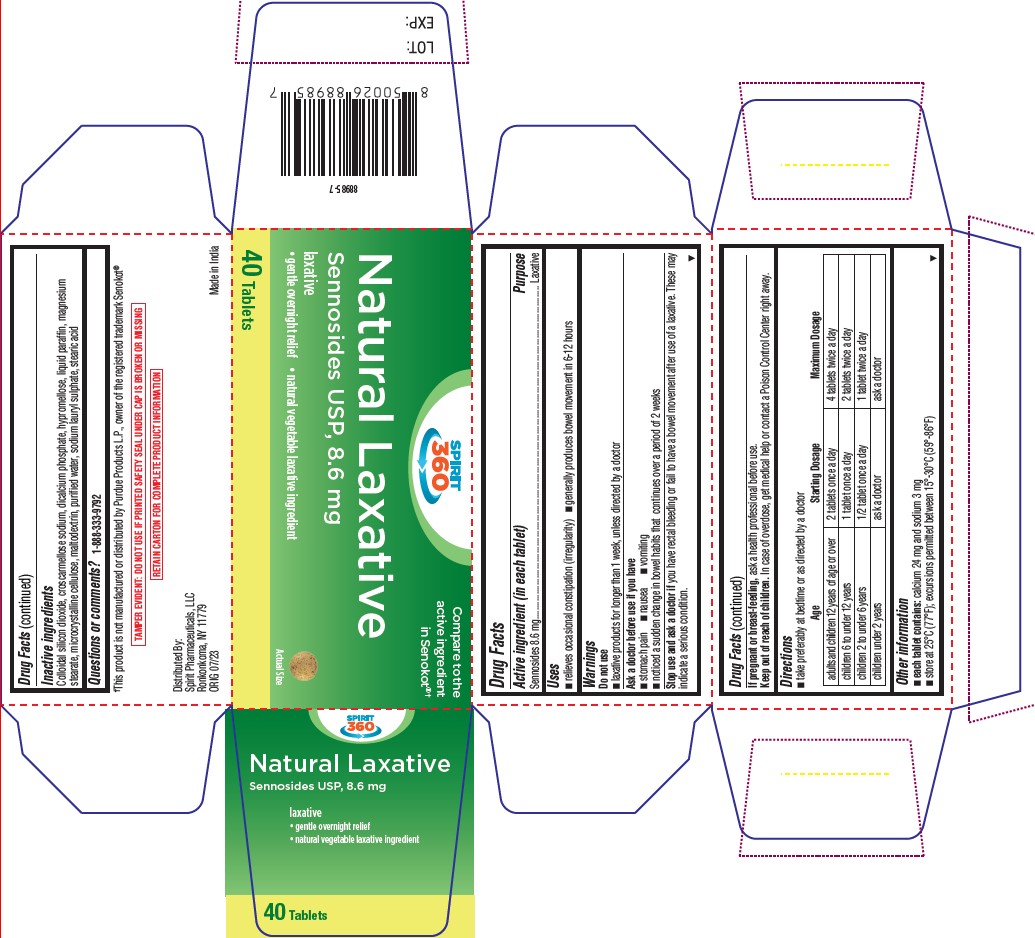
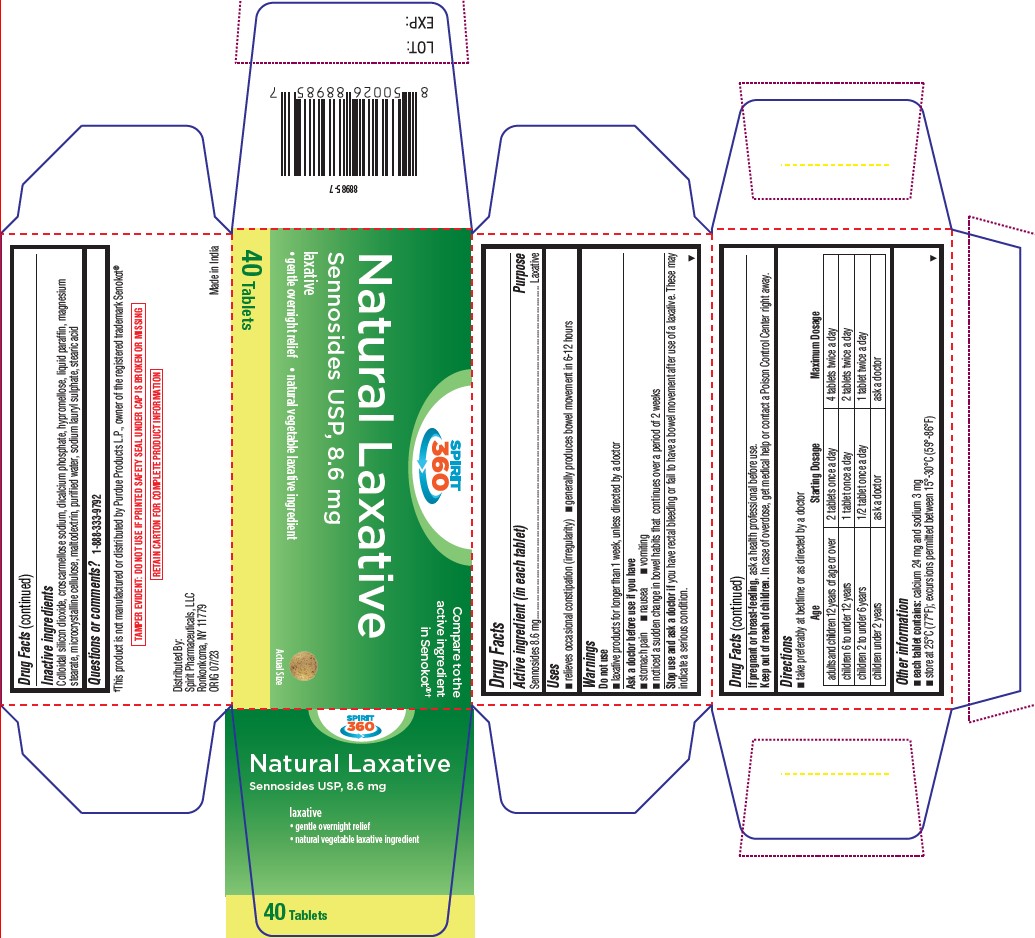
Age Starting Dosage Maximum Dosage adults and children 12 years of age or over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VEGETABLE LAXATIVE
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-5017 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) HYPROMELLOSES (UNII: 3NXW29V3WO) PARAFFIN (UNII: I9O0E3H2ZE) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MALTODEXTRIN (UNII: 7CVR7L4A2D) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code S8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-5017-4 1 in 1 CARTON 11/30/2023 1 40 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 11/29/2023 Labeler - Spirit Pharmaceuticals LLC (179621011)