Label: SACUBITRIL AND VALSARTAN tablet, film coated
- NDC Code(s): 62332-556-10, 62332-556-45, 62332-556-60, 62332-556-91, view more
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SACUBITRIL AND VALSARTAN TABLETS safely and effectively. See full prescribing information for SACUBITRIL AND VALSARTAN TABLETS ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue sacubitril and valsartan tablets as soon as possible (5.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1)
-
1 INDICATIONS AND USAGE1.1 Adult Heart Failure - Sacubitril and valsartan tablets are indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - Sacubitril and valsartan tablet is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to ...
-
3 DOSAGE FORMS AND STRENGTHSSacubitril and valsartan tablets 24 mg/26 mg are white to off white, modified capsule shaped, biconvex film-coated tablets debossed with “725” on one side and “L” on the other side. Sacubitril ...
-
4 CONTRAINDICATIONSSacubitril and valsartan tablets are contraindicated: in patients with hypersensitivity to any component - in patients with a history of angioedema related to previous ACE inhibitor or ARB therapy ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Sacubitril and valsartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include: Angioedema [see Warnings and Precautions (5.2)] Hypotension [see Warnings and Precautions (5.3) ...
-
7 DRUG INTERACTIONS7.1 Dual Blockade of the Renin-Angiotensin-Aldosterone System - Concomitant use of sacubitril and valsartan with an ACE inhibitor is contraindicated because of the increased risk of angioedema ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sacubitril and valsartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and ...
-
10 OVERDOSAGELimited data are available with regard to overdosage in human subjects with sacubitril and valsartan. In healthy volunteers, a single dose of sacubitril and valsartan tablet 583 mg sacubitril/617 ...
-
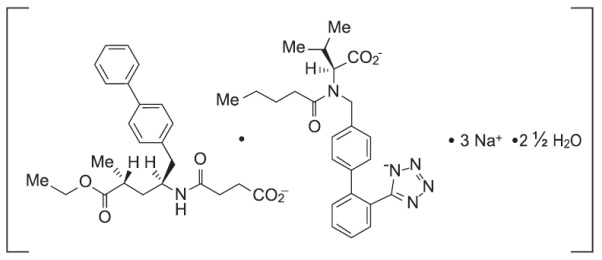
11 DESCRIPTIONSacubitril and valsartan is a combination of a neprilysin inhibitor and an angiotensin II receptor blocker. The active pharmaceutical ingredient used to manufacture sacubitril and valsartan ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sacubitril and valsartan contains a neprilysin inhibitor, sacubitril, and an angiotensin receptor blocker, valsartan. Sacubitril and valsartan inhibits neprilysin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis - Carcinogenicity studies conducted in mice and rats with sacubitril and valsartan did not identify any ...
-
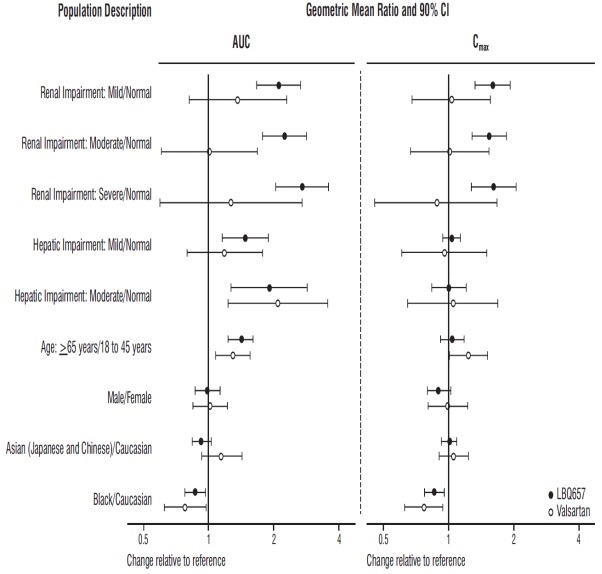
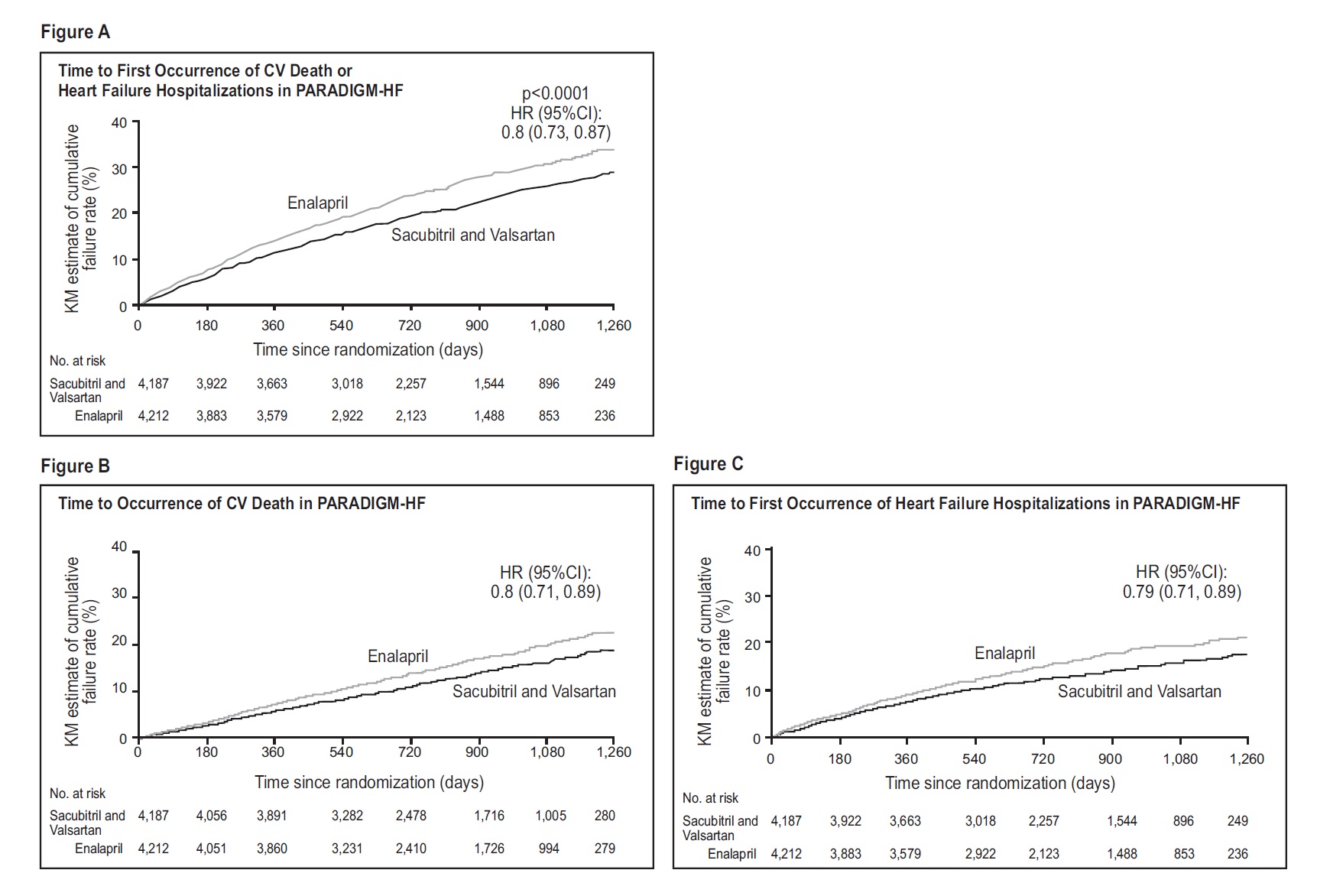
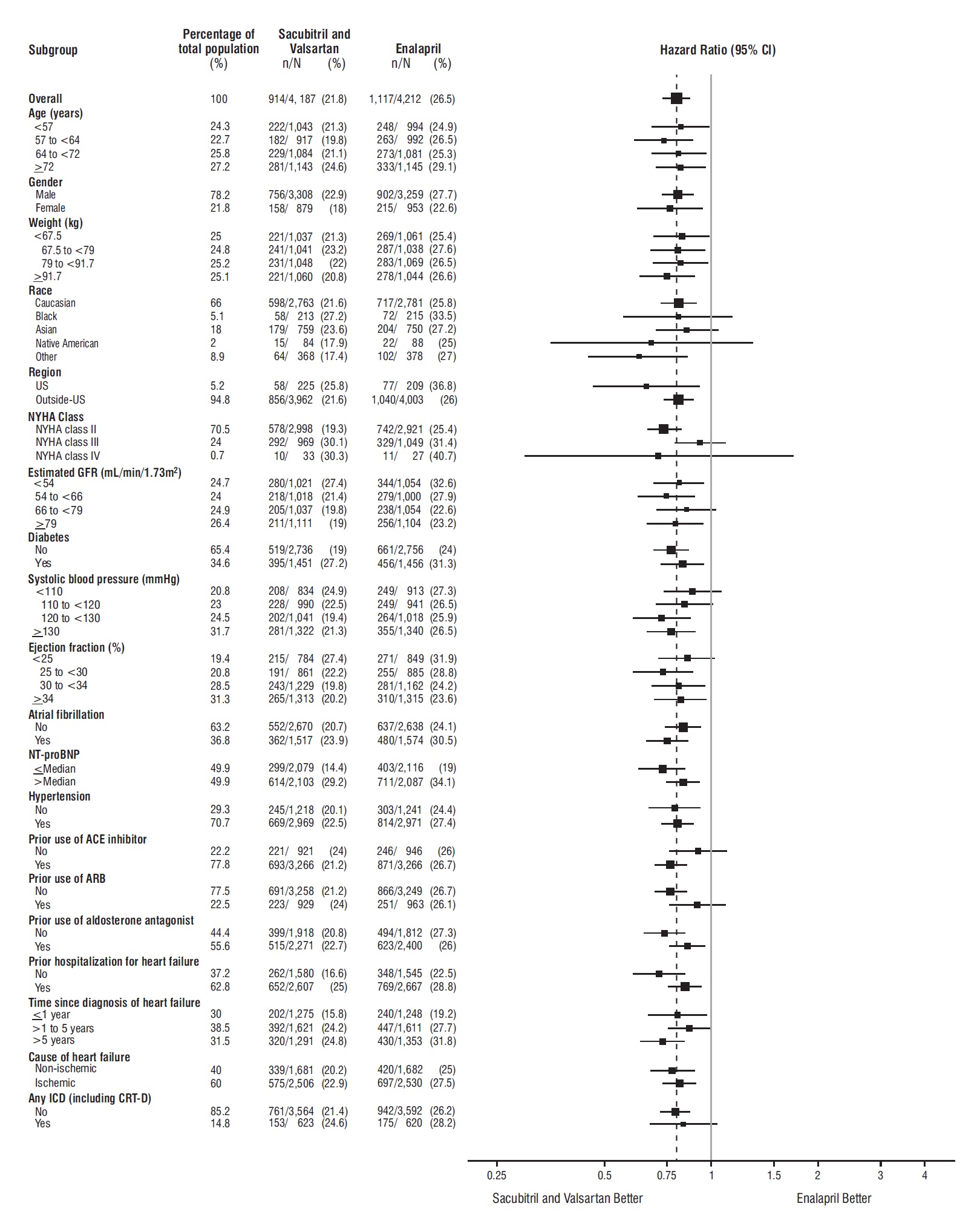
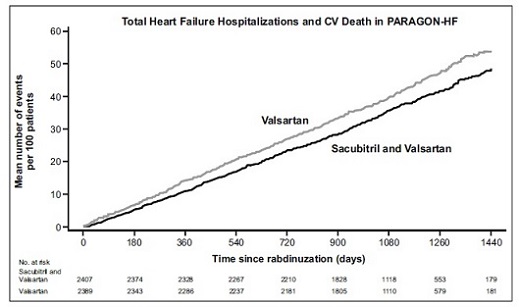
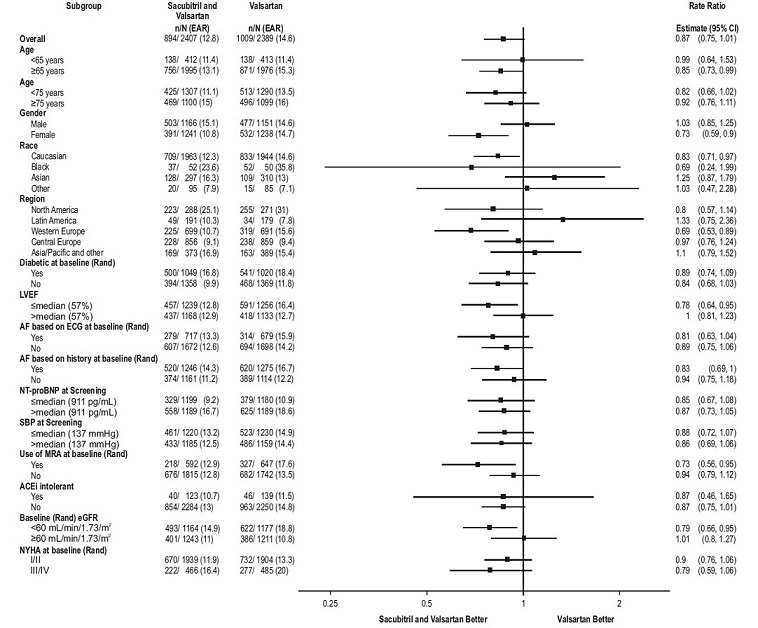
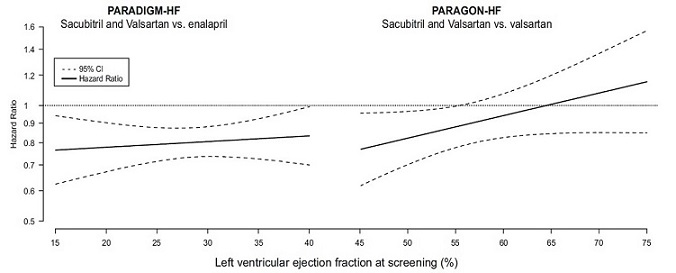
14 CLINICAL STUDIESDosing in clinical trials was based on the total amount of both components of sacubitril and valsartan, i.e., 24/26 mg, 49/51 mg, and 97/103 mg were referred to as 50 mg, 100 mg, and 200 mg ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSacubitril and valsartan tablets 24 mg/26 mg are white to off white, modified capsule shaped, biconvex film-coated tablets debossed with “725” on one side and “L” on the other side. NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of exposure to sacubitril and ...
-
PATIENT PACKAGE INSERTPatient Information - Sacubitril and Valsartan - (sak UE bi tril and val SAR tan) Tablets - What is the most important information I should know about sacubitril and valsartan ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 24 mg/26 mgNDC 62332-556-60 - Sacubitril and - Valsartan Tablets - 24 mg/26 mg - Rx only - 60 Tablets - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 49 mg/51 mgNDC 62332-557-60 - Sacubitril and - Valsartan Tablets - 49 mg/51 mg - Rx only - 60 Tablets - Alembic
-
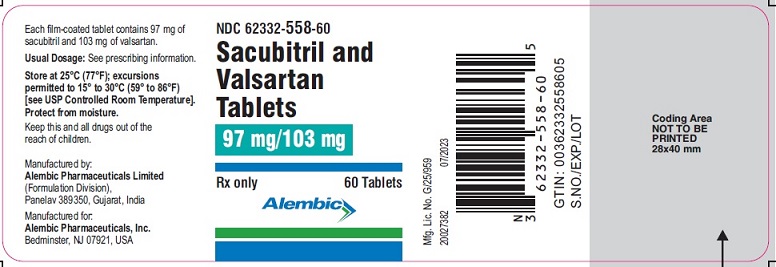
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 97 mg/103 mgNDC 62332-558-60 - Sacubitril and - Valsartan Tablets - 97 mg/103 mg - Rx only - 60 Tablets - Alembic
-
INGREDIENTS AND APPEARANCEProduct Information