Label: GOOD NEIGHBOR PHARMACY HYDROCORTISONE- hydrocortisone ointment
- NDC Code(s): 24385-276-03
- Packager: AmerisourceBergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
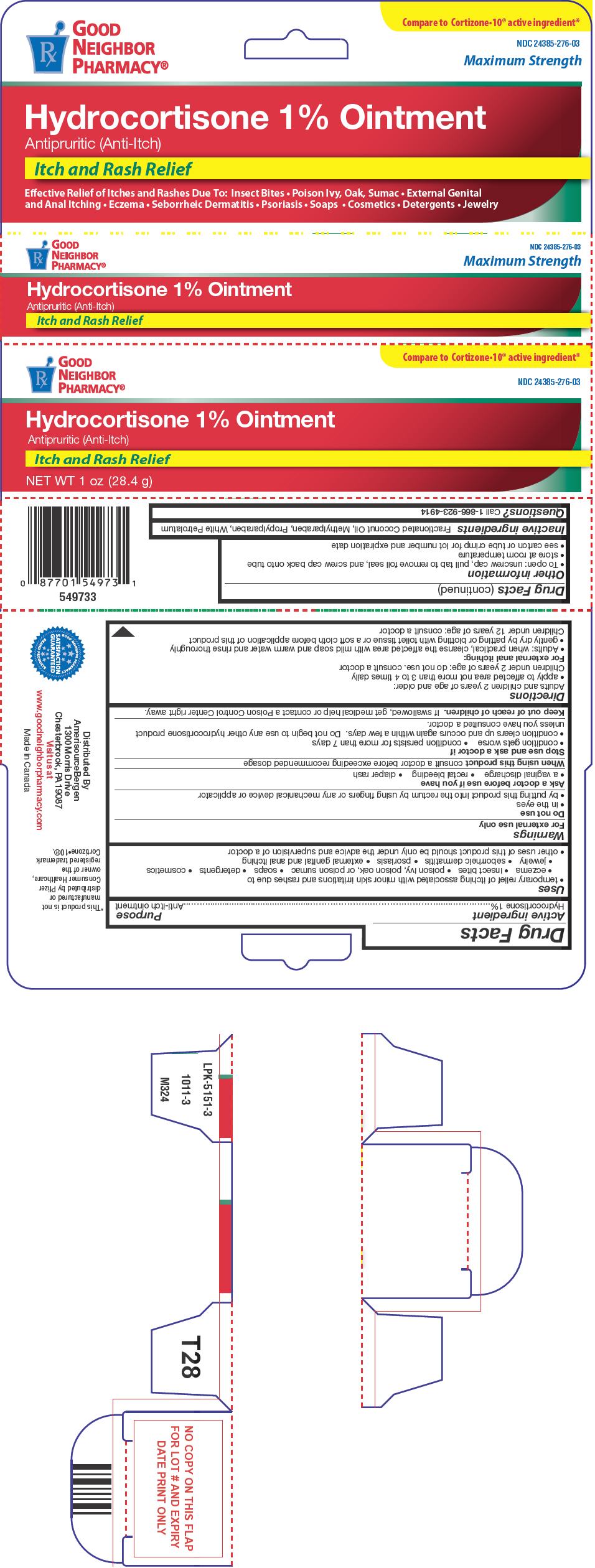
- Active ingredient
- Purpose
-
Uses
- temporary relief of itching associated with minor skin irritations and rashes due to
- eczema
- insect bites
- poison ivy, poison oak, or poison sumac
- soaps
- detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- external genital and anal itching
- other uses of this product should be only under the advice and supervision of a doctor
- temporary relief of itching associated with minor skin irritations and rashes due to
-
Warnings
For external use only
Do not use
- in the eyes
- by putting this product into the rectum by using fingers or any mechanical device or applicator
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
-
INGREDIENTS AND APPEARANCE
GOOD NEIGHBOR PHARMACY HYDROCORTISONE
hydrocortisone ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24385-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24385-276-03 1 in 1 CARTON 10/03/1989 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/03/1989 Labeler - AmerisourceBergen (007914906) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 manufacture(24385-276)