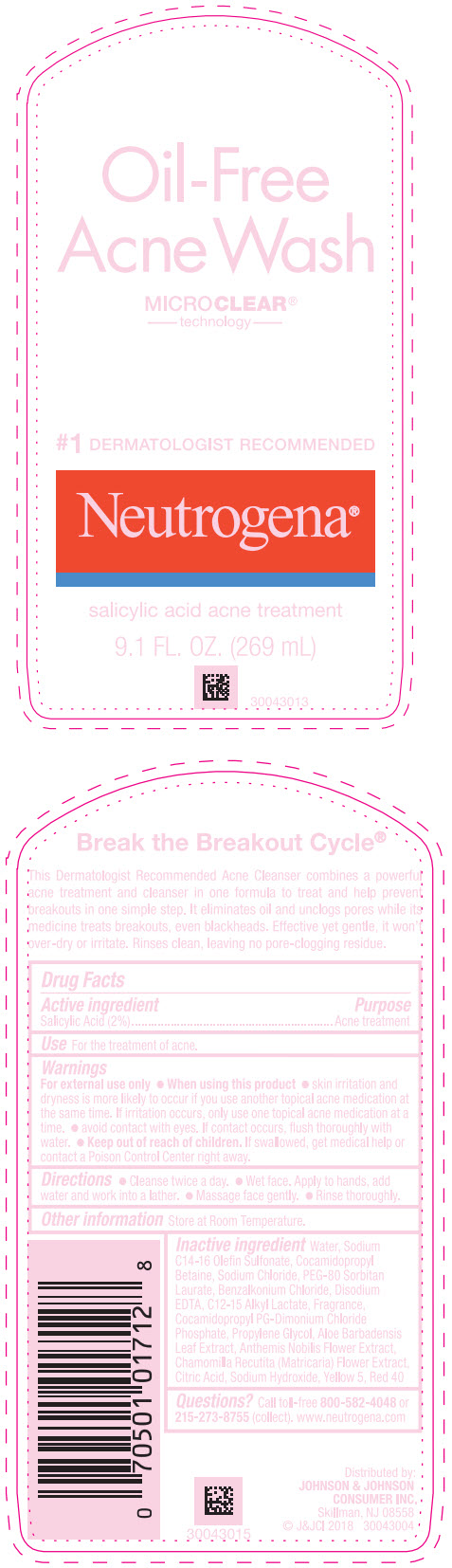
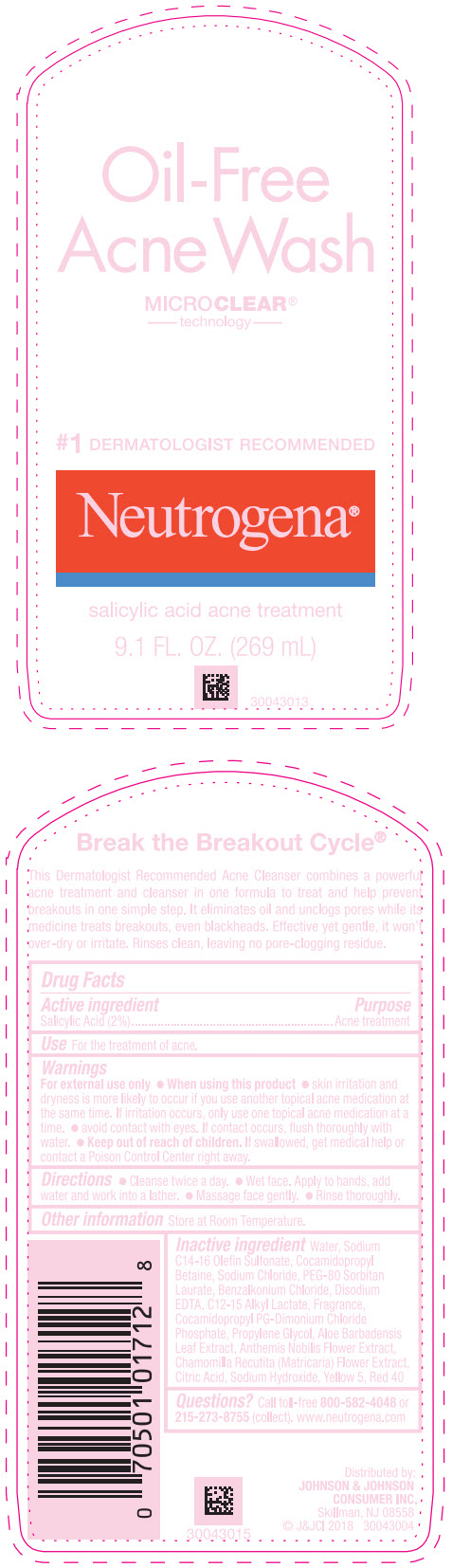
Label: NEUTROGENA OIL FREE ACNE WASH- salicylic acid lotion
- NDC Code(s): 69968-0516-6, 69968-0516-9
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
- Directions
- Other information
-
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Sodium Chloride, PEG-80 Sorbitan Laurate, Benzalkonium Chloride, Disodium EDTA, C12-15 Alkyl Lactate, Fragrance, Cocamidopropyl PG-Dimonium Chloride Phosphate, Propylene Glycol, Aloe Barbadensis Leaf Extract, Anthemis Nobilis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Sodium Hydroxide, Yellow 5, Red 40
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 269 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
NEUTROGENA OIL FREE ACNE WASH
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0516 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-80 SORBITAN LAURATE (UNII: 239B50Y732) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0516-9 269 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2009 2 NDC:69968-0516-6 177 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 02/01/2009 Labeler - Kenvue Brands LLC (118772437)