#1 DERMATOLOGIST RECOMMENDED ACTIVE INGREDIENT†
IMPORTANT INFORMATION ABOUT
-
Minoxidil Topical Aerosol, 5% (Foam)
Hair Regrowth Treatment
-
For Women
-
•
Reactivates Hair Follicles To ...
#1 DERMATOLOGIST RECOMMENDED ACTIVE INGREDIENT†
IMPORTANT INFORMATION ABOUT
Minoxidil Topical Aerosol, 5% (Foam)
Hair Regrowth Treatment
For Women
- •
-
Reactivates Hair Follicles To Stimulate Regrowth
- •
-
Clinically Proven to Regrow Hair
- •
-
Once-a-Day Foam For Women
UNSCENTED
SAVE THIS LEAFLET FOR FUTURE REFERENCE
Please read this leaflet carefully. It will help you understand how to use Minoxidil Topical Aerosol, 5% (For Women) and what to expect from its use. It takes time to regrow hair. Results may occur at 3 months with once daily usage. For some women, you may need to use this product for at least 6 months before you see results. If you have any questions after reading this leaflet, or anytime while using Minoxidil Topical Aerosol, 5% (For Women), you should ask your health care professional or call us at 1-800-719-9260.
WHAT IS MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Minoxidil Topical Aerosol, 5% (For Women) is a white foam containing 5% minoxidil for use only on the scalp to help regrow hair in women.
WHO MAY USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Minoxidil Topical Aerosol, 5% (For Women) may be appropriate for you if you are an adult who is at least 18 years old and experiencing gradually thinning hair or gradual hair loss on the top of your scalp. The common hereditary thinning or hair loss process begins slowly and may become noticeable only after years of gradual loss.
Minoxidil Topical Aerosol, 5% (For Women) is for general thinning of hair on the top of the scalp as shown. Minoxidil Topical Aerosol, 5% (For Women) has been shown to regrow hair in women with the degrees of hair loss shown. If women have more hair loss than shown, Minoxidil Topical Aerosol, 5% (For Women) may not work.
Many of those experiencing hair loss have other family members with gradual thinning of hair or hair loss. If there is no family history of gradual thinning or gradual hair loss, or hair loss is patchy, see your doctor.
WHO SHOULD NOT USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Minoxidil Topical Aerosol, 5% (For Women) will not prevent or improve hair loss related to pregnancy, the use of some prescription and non-prescription medications, certain severe nutritional problems (very low body iron; excessive vitamin A intake), the recently discontinued use of birth control pills, low thyroid states (hypothyroidism), chemotherapy, or diseases which cause scarring of the scalp. Also, Minoxidil Topical Aerosol, 5% (For Women) will not improve hair loss due to:
- •
- Damage from the use of hair care products which cause scarring or deep burns of the scalp.
- •
- Hair grooming methods such as cornrowing or ponytails which require pulling the hair tightly back from the scalp.
Do not use if you are not sure of the reason for your hair loss.
WILL MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) WORK FOR ME?
The amount of hair regrowth is different for each person. Not everyone will respond to Minoxidil Topical Aerosol, 5% (For Women). The response to Minoxidil Topical Aerosol, 5% (For Women) cannot be predicted. No one will be able to grow back all their hair.
To see your best results with Minoxidil Topical Aerosol, 5% (For Women), make sure you apply the product directly to the scalp once daily. For some women, Minoxidil Topical Aerosol, 5% (For Women) may not work.
HOW SOON CAN I EXPECT RESULTS FROM USING MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Since normal hair usually grows only 1/2 to 1 inch per month, hair regrowth with Minoxidil Topical Aerosol, 5% (For Women) also takes time. Generally, new hair growth is slow for a Minoxidil Topical Aerosol, 5% (For Women) user. Results may be seen as early as 3 months with once daily use. For some women, it may take at least 6 months for results to be seen. If you do not see any results after 6 months, stop using Minoxidil Topical Aerosol, 5% (For Women) and seek the advice of your doctor.
When you first begin to use Minoxidil Topical Aerosol, 5% (For Women), your hair loss may continue for up to 2 weeks. This increase in hair loss is expected and is temporary. However, if it continues after two weeks, see your physician.
IF MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) IS WORKING, WHAT WILL THE HAIR LOOK LIKE?
At first, hair growth may be soft, downy, and colorless hairs. After further use, the new hairs should be the same color and thickness as the other hairs on your scalp.
HOW LONG DO I NEED TO USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
If you respond to Minoxidil Topical Aerosol, 5% (For Women), you need to use it once daily to keep and continue the hair regrowth. Up to 3 months of usage may be needed to see your best results from Minoxidil Topical Aerosol, 5% (For Women).
WHAT HAPPENS IF I COMPLETELY STOP USING MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)? WILL I KEEP THE NEW HAIR?
Continuous use of Minoxidil Topical Aerosol, 5% (For Women) is needed to maintain hair regrowth. If you stop using Minoxidil Topical Aerosol, 5% (For Women), the normal hair loss process will start again. You will probably lose your newly regrown hair in three to four months.
HOW DO I USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
For best results, apply half a capful once a day directly to the scalp in the hair loss area. Using more than the recommended amount will not improve results. Each can should last two months, if used as directed. Never take this product by mouth or apply to other parts of the body. Please refer to the “Directions for Use” section of this leaflet.
WHEN DO I USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Apply Minoxidil Topical Aerosol, 5% (For Women) once daily. If you apply at night, allow application to dry completely before going to bed.
WHAT IF I FORGET TO USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) OR MISS A DOSE?
If you miss one daily dose of Minoxidil Topical Aerosol, 5% (For Women), just continue with your next dose. You should not make up for missed doses.
CAN I USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) MORE THAN ONCE A DAY? WILL IT WORK FASTER, BETTER?
No. Minoxidil Topical Aerosol, 5% (For Women) will not work faster or better if used more than once a day. More frequent use or larger doses have not been shown to speed up hair growth and may increase your chances of side effects.
WHAT KIND OF SHAMPOO SHOULD I USE WITH MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
If you wash your scalp before applying Minoxidil Topical Aerosol, 5% (For Women), use a mild shampoo.
CAN I USE HAIR SPRAYS, MOUSSES, CONDITIONERS, GELS, ETC.?
Yes. There is no reason to change your usual hair care routine when using Minoxidil Topical Aerosol, 5% (For Women). However, you should apply Minoxidil Topical Aerosol, 5% (For Women) first and wait for it to dry before applying your styling aids.
CAN I HAVE MY HAIR COLORED OR PERMED OR USE HAIR RELAXERS WHILE USING MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Yes. We have no evidence that coloring, perming, or using relaxers on your hair change the effect of Minoxidil Topical Aerosol, 5% (For Women). However, because the use of a permanent wave and hair color can cause scalp irritation on certain people, we recommend the following precautions:
- 1.
- To avoid possible scalp irritation, you should make sure all of the Minoxidil Topical Aerosol, 5% (For Women) has been washed off the hair and scalp before using color or perm chemicals.
- 2.
- For best results, do not apply Minoxidil Topical Aerosol, 5% (For Women) on the same day that you use a chemical treatment on your hair.
- 3.
- Do not use Minoxidil Topical Aerosol, 5% (For Women) for 24 hours after using any chemicals to make sure your scalp has not been irritated by the perm or color treatment. If no irritation occurs, continue use of Minoxidil Topical Aerosol, 5% (For Women) as usual.
- 4.
- Simply restart your normal Minoxidil Topical Aerosol, 5% (For Women) routine. There is no need to use more Minoxidil Topical Aerosol, 5% (For Women) to make up for missed applications.
Missing one day of Minoxidil Topical Aerosol, 5% (For Women) will not affect your hair regrowth results.
CAN I APPLY MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) AND WASH MY HAIR AN HOUR LATER?
No. For Minoxidil Topical Aerosol, 5% (For Women) to work best, you should allow Minoxidil Topical Aerosol, 5% (For Women) to remain on the scalp for about 4 hours before washing.
DIRECTIONS FOR USE
- •
- Please familiarize yourself with the instructions below in order to help make your product application a success.
- •
- This product should be used ONCE DAILY, EVERY DAY.
- •
- To be effective, it is important to apply the product DIRECTLY TO YOUR SCALP and NOT TO YOUR HAIR so that it can easily get to your hair follicles to help regrow your hair.
- •
- There is no need to shampoo your hair before using the product. If you wish to shampoo your hair before applying Minoxidil Topical Aerosol, 5% (For Women), towel dry your hair so that the skin on the scalp is dry.
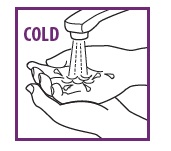
1: PREP HANDS WITH COLD WATER
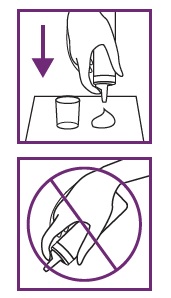
Rinse your fingers in coldwater and dry them. Note: Foam will melt on contact with warm surfaces.
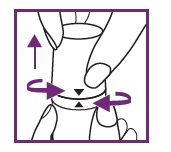
2: OPEN CHILD RESISTANT CAP
Be sure to align arrow on the cap with arrow on white ring. Tilt cap back and pull off.
3: DISPENSE VERTICALLY ONTO A COLD SURFACE
Hold the can straight upside down. If you hold the can at an angle, foam may not dispense properly. Press nozzle to dispense half a capful of foam onto cold surface (e.g. dish, or cold hands).
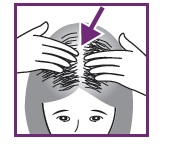
4:APPLY TO SCALP, NOT HAIR
Part your hair to expose hair loss area. Massage foam into scalp, not hair. Repeat until all hair loss areas have been covered.
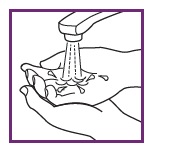
5: CLOSE CAP AND WASH HANDS
Snap cap back into place. Be sure arrows do not line up, so cap remains child resistant. Wash hands and any surface thoroughly after use.
ARE THERE ANY SPECIAL WARNINGS ABOUT THE USE OF MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Warnings:
For external use only.
Extremely Flammable: Avoid fire, flame, or smoking during or immediately following application.
DO NOT USE IF
- •
- your degree of hair loss is more than that shown on the other side of this leaflet, because this product may not work for you
- •
- you have no family history of hair loss
- •
- your hair loss is sudden and/or patchy
- •
- your hair loss is associated with childbirth
- •
- you do not know the reason for your hair loss
- •
- you are under 18 years of age. Do not use on babies and children.
- •
- your scalp is red, inflamed, infected, irritated, or painful
- •
- you use other medicines on the scalp
Ask a doctor before use if you have heart disease.
WHEN USING THIS PRODUCT
- •
- do not use more than directed
- •
- do not apply on other parts of the body
- •
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- •
- some people have experienced changes in hair color and/or texture
- •
- it takes time to regrow hair. Results may occur in 3 months with once daily use. For some women you may need to use this product once daily for at least 6 months before you see results.
- •
- the amount of hair regrowth is different for each person. This product will not work for everyone.
STOP USE AND ASK A DOCTOR IF
- •
- chest pain, rapid heartbeat, faintness, or dizziness occurs
- •
- sudden, unexplained weight gain occurs
- •
- your hands or feet swell
- •
- scalp irritation or redness occurs
- •
- unwanted facial hair growth occurs
- •
- you do not see hair regrowth in 6 months
May be harmful if used when pregnant or breast-feeding.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
WHAT ARE THE MOST COMMON SIDE EFFECTS WITH MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
The most common side effects are itching and other skin irritations of the treated area of the scalp. Minoxidil Topical Aerosol, 5% (For Women) contains alcohol, which would cause burning or irritation of the eyes or sensitive skin areas. If Minoxidil Topical Aerosol, 5% (For Women) accidentally gets into these areas, rinse with large amounts of cool tap water. Stop using Minoxidil Topical Aerosol, 5% (For Women) and contact your doctor if irritation persists.
CAN MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) PRODUCE UNWANTED HAIR?
Unwanted hair growth has been reported on the face and on other parts of the body with Minoxidil Topical Aerosol, 5% (For Women) use. The unwanted hair growth may be caused by the transfer of Minoxidil Topical Aerosol, 5% (For Women) to areas other than the scalp, or by absorption into the circulatory system of low levels of the active ingredient, or by a medical condition not related to the use of Minoxidil Topical Aerosol, 5% (For Women).
If you experience unwanted hair, discontinue using Minoxidil Topical Aerosol, 5% (For Women) and see your doctor for recommendations about appropriate treatment. After stopping use of Minoxidil Topical Aerosol, 5% (For Women), the unwanted hair, if caused by the use of Minoxidil Topical Aerosol, 5% (For Women), should go away over time. You can take steps to decrease the chances for unwanted hair growth:
- 1.
- Limit the application of Minoxidil Topical Aerosol, 5% (For Women) only to the scalp,
- 2.
- If you use the hands to apply Minoxidil Topical Aerosol, 5% (For Women), wash your hands thoroughly afterwards, and
- 3.
- Allow sufficient drying time before going to bed (usually 2 to 4 hours before going to bed after applying Minoxidil Topical Aerosol, 5% (For Women)).
CAN I USE MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN) FOR BALDNESS OR HAIR LOSS IN BABIES AND CHILDREN?
No. Minoxidil Topical Aerosol, 5% (For Women) must not be used to treat baldness or hair loss in babies or children.
WHAT FACTORS MAY INCREASE THE RISK OF SERIOUS SIDE EFFECTS WITH MINOXIDIL TOPICAL AEROSOL, 5% (FOR WOMEN)?
Minoxidil Topical Aerosol, 5% (For Women) should be applied only to the scalp. The risk of side effects may be greater when Minoxidil Topical Aerosol, 5% (For Women) is applied to other parts of the body.
If you have any other questions, ask your health care professional or call us at 1-800-719-9260.
Store at 20° to 25°C (68° to 77°F).
Contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120°F (49°C).
Save this leaflet for future reference.
†Of U.S. Physicians surveyed by an independent market research firm.
Made in Israel
Distributed By
Perrigo®
Allegan, MI 49010
Rev 08-21
: 12900 00 J4
Close