LEVORA
-
®0.15/30-28
-
(levonorgestrel 0.15 mg and ethinyl estradiol 0.03 mg tablets)
What is the most important information I should know about LEVORA 0.15/30-28?
Do not use ...
LEVORA
®0.15/30-28
(levonorgestrel 0.15 mg and ethinyl estradiol 0.03 mg tablets)
What is the most important information I should know about LEVORA 0.15/30-28?
Do not use LEVORA 0.15/30-28 if you smoke cigarettes and are over 35 years old.Smoking increases your risk of serious cardiovascular side effects from hormonal birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What is LEVORA 0.15/30-28?
LEVORA 0.15/30-28 is a birth control pill (oral contraceptive) used by women to prevent pregnancy.
How does LEVORA 0.15/30-28 work for contraception?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
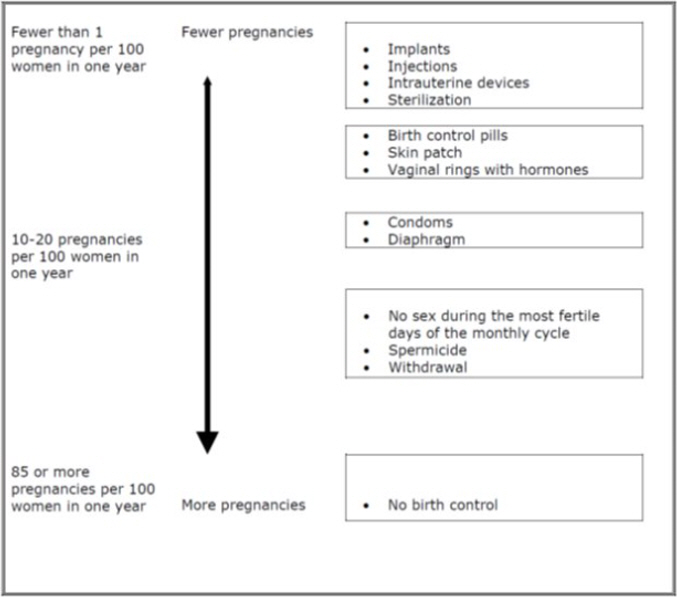
Based on the results of clinical studies, about 1 to 5 out of 100 women may get pregnant during the first year they use LEVORA 0.15/30-28.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Who should not take LEVORA 0.15/30-28?
Do not take LEVORA 0.15/30-28 if you:
- smoke and are over 35 years of age
- had blood clots in your arms, legs, lungs, or eyes
- had a problem with your blood that makes it clot more than normal
- have certain heart valve problems or irregular heart beat
- had a stroke
- had a heart attack
- have high blood pressure that cannot be controlled by medicine
- have diabetes with kidney, eye, nerve, or blood vessel damage
- have certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or any migraine headaches if you are over 35 years of age
- had breast cancer or any cancer that is sensitive to female hormones
- have liver problems, including liver tumors
- have any unexplained vaginal bleeding
- are pregnant
- take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme "alanine aminotransferase" (ALT) in the blood.
If any of these conditions happen while you are taking LEVORA 0.15/30-28, stop taking LEVORA 0.15/30-28 right away and talk to your healthcare provider. Use non-hormonal contraception when you stop taking LEVORA 0.15/30-28.
What should I tell my healthcare provider before taking LEVORA 0.15/30-28?
Tell your healthcare provider if you:
- are pregnant or think you may be pregnant
- are depressed now or have been depressed in the past
- had yellowing of your skin or eyes (jaundice) caused by pregnancy (cholestasis of pregnancy)
- are breastfeeding or plan to breastfeed. LEVORA 0.15/30-28 may decrease the amount of breast milk you make. A small amount of the hormones in LEVORA 0.15/30-28 may pass into your breast milk. Talk to your healthcare provider about the best birth control method for you while breastfeeding.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins and herbal supplements.
LEVORA 0.15/30-28 may affect the way other medicines work, and other medicines may affect how well LEVORA 0.15/30-28 works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take LEVORA 0.15/30-28?
Read the Instructions for Use at the end of this Patient Information.
What are the possible serious side effects of LEVORA 0.15/30-28?
- Like pregnancy, LEVORA 0.15/30-28 may cause serious side effects, including blood clots in your lungs, heart attack, or a stroke that may lead to death. Some other examples of serious blood clots include blood clots in the legs or eyes.
Serious blood clots can happen especially if you smoke, are obese, or are older than 35 years of age. Serious blood clots are more likely to happen when you:
- first start taking birth control pills
- restart the same or different birth control pills after not using them for a month or more
Call your healthcare provider or go to a hospital emergency room right away if you have:
- leg pain that will not go away
- sudden sever shortness of breath
- sudden change in vision or blindness
- chest pain
- a sudden, severe headache unlike your usual headaches
- weakness or numbness in your arm or leg
- trouble speaking
Other serious side effects include:
-
liver problems, including:
- rare liver tumors
- jaundice (cholestasis), especially if you previously had cholestasis of pregnancy. Call your healthcare provider if you have yellowing of your skin or eyes.
-
high blood pressure.You should see your healthcare provider for a yearly check of your blood pressure.
-
gallbladder problems
-
changes in the sugar and fat (cholesterol and triglycerides) levels in your blood
-
new or worsening headaches, including migraine headaches
-
irregular or unusual vaginal bleeding and spotting between your menstrual periods, especially during the first 3 months of taking LEVORA 0.15/30-28.
-
depression
-
possible cancer in your breast and cervix
-
swelling of your skin especially around your mouth, eyes, and in your throat (angioedema).Call your healthcare provider if you have a swollen face, lips, mouth tongue or throat, which may lead to difficulty swallowing or breathing. Your chance of having angioedema is higher if you have a history of angioedema.
-
dark patches of skin around your forehead, nose, cheeks and around your mouth, especially during pregnancy (chloasma).Women who tend to get chloasma should avoid spending a long time in sunlight, tanning booths, and under sun lamps while taking LEVORA 0.15/30-28. Use sunscreen if you have to be in the sunlight.
What are the most common side effects of oral contraceptives?
- nausea
- vomiting
- bleeding between menstrual periods
- weight gain
- breast tenderness
- difficulty wearing contact lenses
These are not all the possible side effects of LEVORA 0.15/30-28. For more information, ask your healthcare provider or pharmacist.
You may report side effects to the FDA at 1-800-FDA-1088.
What else should I know about taking LEVORA 0.15/30-28?
- If you are scheduled for any lab tests, tell your healthcare provider you are taking LEVORA 0.15/30-28. Certain blood tests may be affected by LEVORA 0.15/30-28.
- LEVORA 0.15/30-28 does not protect against HIV-infection (AIDS) and other sexually transmitted infections.
How should I store LEVORA 0.15/30-28?
- Store LEVORA 0.15/30-28 at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light.
General information about the safe and effective use of LEVORA 0.15/30-28.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LEVORA 0.15/30-28 for a condition for which it was not prescribed. Do not give LEVORA 0.15/30-28 to other people, even if they have the same symptoms that you have.
This Patient Information Leaflet summarizes the most important information about LEVORA 0.15/30-28. You can ask your pharmacist or healthcare provider for information about LEVORA 0.15/30-28 that is written for health professionals
.
For more information, call 1-888-375-3784.
Does hormonal birth control cause cancer?
It is not known if hormonal birth control pills causes breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
There may be slight increases in the risk of breast cancer among current users of hormonal birth control pills with longer duration of use of 8 years or more.
What if I want to become pregnant?
You may stop taking the pill whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the pill.
What should I know about my period when taking LEVORA 0.15/30-28?
Your periods may be lighter and shorter than usual. Some women may miss a period. Irregular vaginal bleeding or spotting may happen while you are taking LEVORA 0.15/30-28, especially during the first few months of use. This usually is not a serious problem. It is important to continue taking your pills on a regular schedule to prevent a pregnancy.
What are the ingredients in LEVORA 0.15/30-28?
Active ingredients: Each white pill contains levonorgestrel and ethinyl estradiol.
Inactive ingredients:
White pills: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone.
Peach pills: FD&C Yellow No. 6 Lake, Lactose Anhydrous, Lactose Monohydrate, Magnesium Stearate and Microcrystalline Cellulose.
Distributed by:
Dr. Reddy’s Laboratories Inc.
Princeton NJ08540
Rev.04/2024
Product of Canada
Close