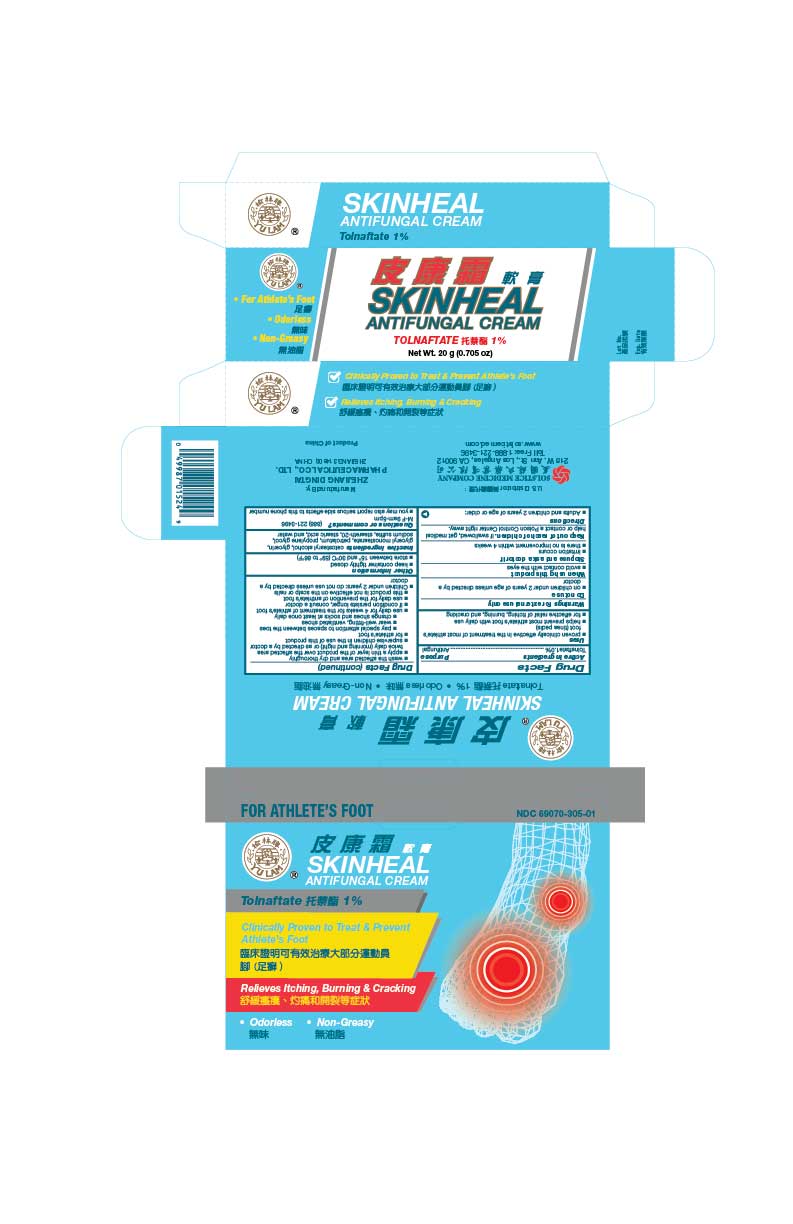
Label: SKINHEAL ANTIFUNGAL- tolnaftate cream
- NDC Code(s): 69070-305-01
- Packager: Zhejiang Dingtai Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults and children 2 years of age or older:
wash the affected area and dry thoroughly
apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor
supervise children in the use of this product
for athlete’s foot
pay special attention to spaces between the toes
wear well-fitting, ventilated shoes
change shoes and socks at least once daily
use daily for 4 weeks for the treatment of athlete’s foot and ringworm
if condition persists longer, consult a doctor
use daily for the prevention of athlete’s foot
this product is not effective on the scalp or nails
Children under 2 years: do not use unless directed by a doctor - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINHEAL ANTIFUNGAL
tolnaftate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69070-305 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM SULFITE (UNII: VTK01UQK3G) STEARETH-20 (UNII: L0Q8IK9E08) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69070-305-01 1 in 1 BOX 12/10/2018 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part333C 12/10/2018 Labeler - Zhejiang Dingtai Pharmaceutical Co., Ltd (420598724) Establishment Name Address ID/FEI Business Operations Zhejiang Dingtai Pharmaceutical Co., Ltd 420598724 manufacture(69070-305)