Label: NICARDIPINE HYDROCHLORIDE injection
- NDC Code(s): 69097-007-22, 69097-007-45, 69097-008-22, 69097-008-45
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NICARDIPINE HYDROCHLORIDE IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for NICARDIPINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Nicardipine hydrochloride in 0.9% sodium chloride injection is indicated for the short-term treatment of hypertension when oral therapy is not feasible or ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Information - Individualize dosing based on the severity of hypertension and the response of the patient during dosing. Monitor blood pressure and heart rate both during and after ...
-
3 DOSAGE FORMS AND STRENGTHSNicardipine hydrochloride in 0.9% sodium chloride injection is available in the following presentations: 20 mg nicardipine hydrochloride in 200 mL 0.9% sodium chloride injection (0.1 mg/mL) in ...
-
4 CONTRAINDICATIONS4.1 Advanced Aortic Stenosis - Do not use nicardipine in patients with advanced aortic stenosis because of the afterload reduction effect of nicardipine. Reduction of diastolic pressure in these ...
-
5 WARNINGS AND PRECAUTIONS5.1 Excessive Pharmacologic Effects - In administrating nicardipine, close monitoring of blood pressure and heart rate is required. Nicardipine may occasionally produce symptomatic hypotension ...
-
6 ADVERSE REACTIONS6.1 Adverse Reactions Observed in Clinical Trials - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot ...
-
7 DRUG INTERACTIONS7.1 Antihypertensive Agents - Since nicardipine hydrochloride injection may be administered to patients already being treated with other medications, including other antihypertensive agents ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There are no adequate and well-controlled studies of nicardipine use in pregnant women. There are limited human data in pregnant women with pre-eclampsia and preterm labor. In ...
-
10 OVERDOSAGESeveral overdosages with orally administered nicardipine have been reported. One adult patient allegedly ingested 600 mg of nicardipine immediate release capsules, and another patient, 2160 mg of ...
-
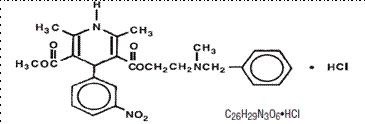
11 DESCRIPTIONNicardipine hydrochloride is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker). Nicardipine hydrochloride is a dihydropyridine derivative with IUPAC (International ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nicardipine inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle without changing serum calcium concentrations. The contractile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Rats treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of 5, 15, or 45 mg/kg/day) for ...
-
14 CLINICAL STUDIESEffects in Hypertension - In patients with mild-to-moderate chronic stable essential hypertension, nicardipine hydrochloride injection (0.5 to 4 mg/hr) produced dose-dependent decreases in blood ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Nicardipine hydrochloride in 0.9% sodium chloride injection is available in packages as follows: NDC - Strength - Packaged - 69097-007-45 - 20 mg in 200 ...
-
PRINCIPAL DISPLAY PANEL-BAG labelsNDC 69097-007-45Rx Only - USE IMMEDIATELY ONCE REMOVED FROM THE OVERWRAP - niCARdipine Hydrochloride - in 0.9% Sodium Chloride Injection - 20 mg in 200 mL - (0.1 mg/mL) For Intravenous ...
-
INGREDIENTS AND APPEARANCEProduct Information