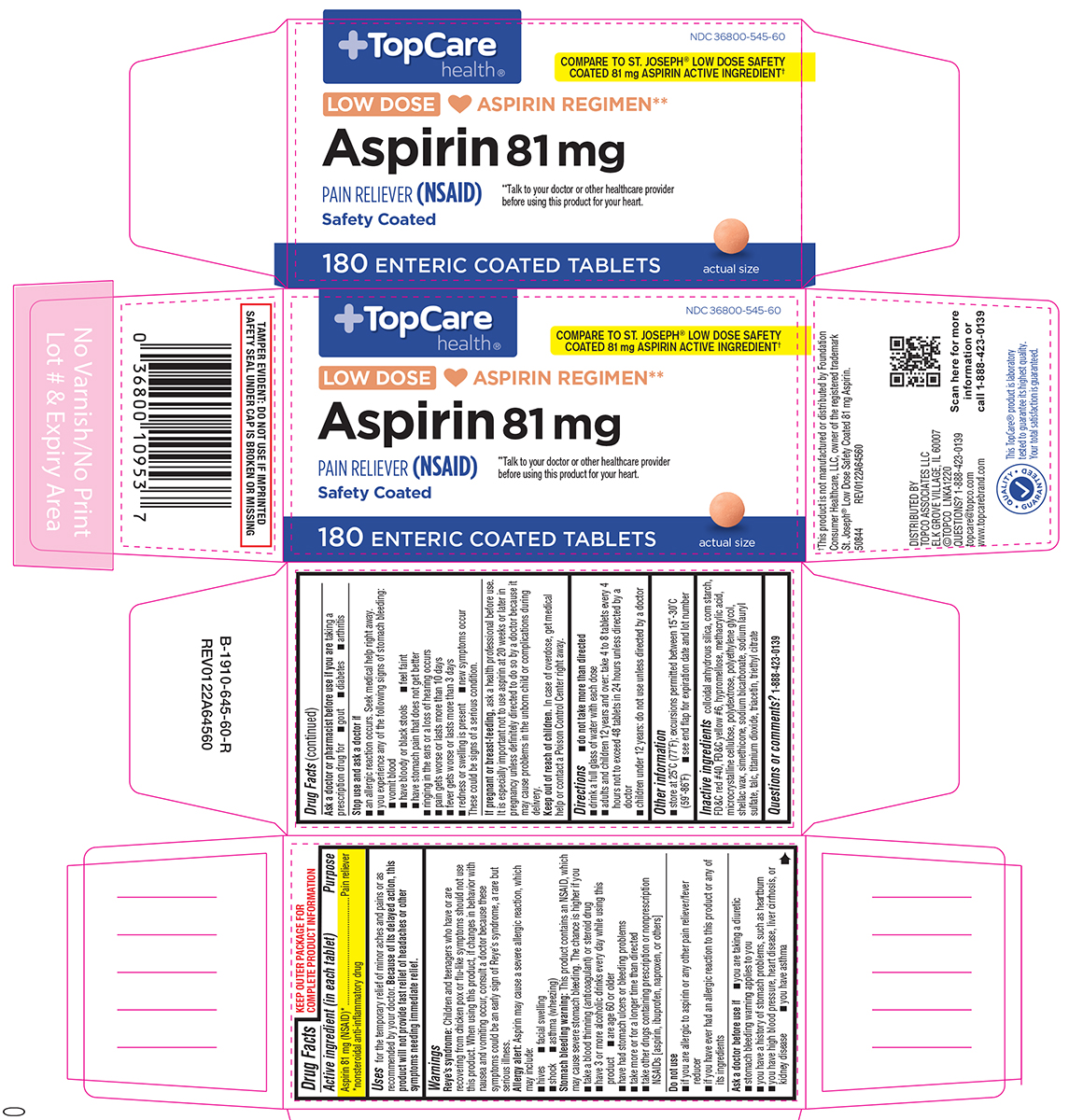
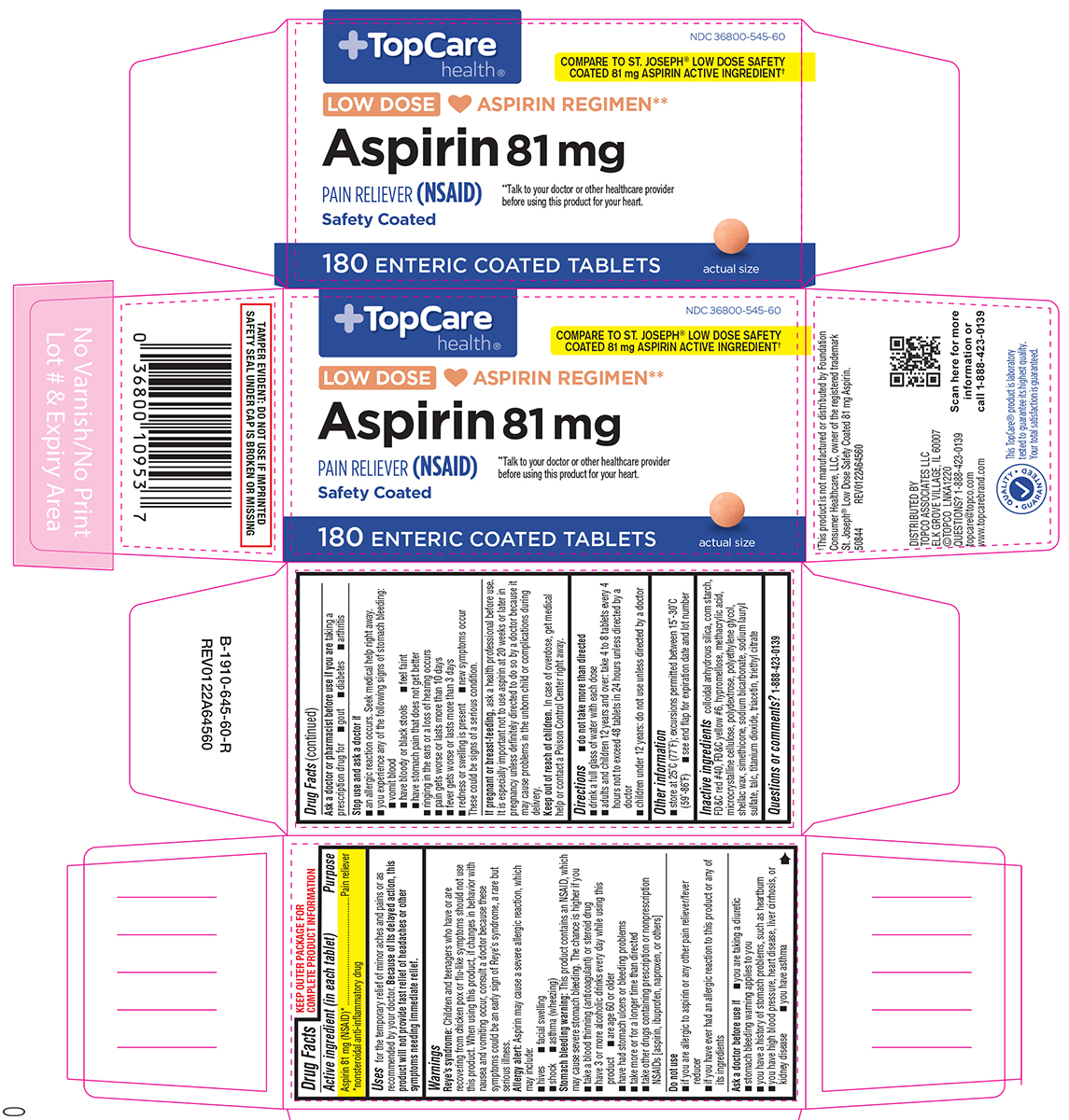
Label: ASPIRIN tablet, delayed release
- NDC Code(s): 36800-545-60
- Packager: Topco Associates, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Aspirin 81 mg (NSAID*) *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever
-
Uses
for the temporary relief of minor aches and pains or as recommended by your doctor
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea ...
-
Directions
do not take more than directed - drink a full glass of water with each dose - adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours ...
-
Other information
store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF) see end flap for expiration date and lot number
-
Inactive ingredients
colloidal anhydrous silica, corn starch, FD&C red #40, FD&C yellow #6, hypromellose, methacrylic acid, microcrystalline cellulose, polydextrose, polyethylene glycol, shellac wax, simethicone ...
-
Questions or comments?
1-888-423-0139
-
Principal Display Panel
NDC 36800-545-60 - TopCare - health® COMPARE TO THE ACTIVE INGREDIENT - IN ST. JOSEPH® LOW DOSE ASPIRIN† LOW DOSE ♥ ASPIRIN REGIMEN** Aspirin 81 mg - PAIN RELIEVER (NSAID) Safety ...
-
INGREDIENTS AND APPEARANCEProduct Information