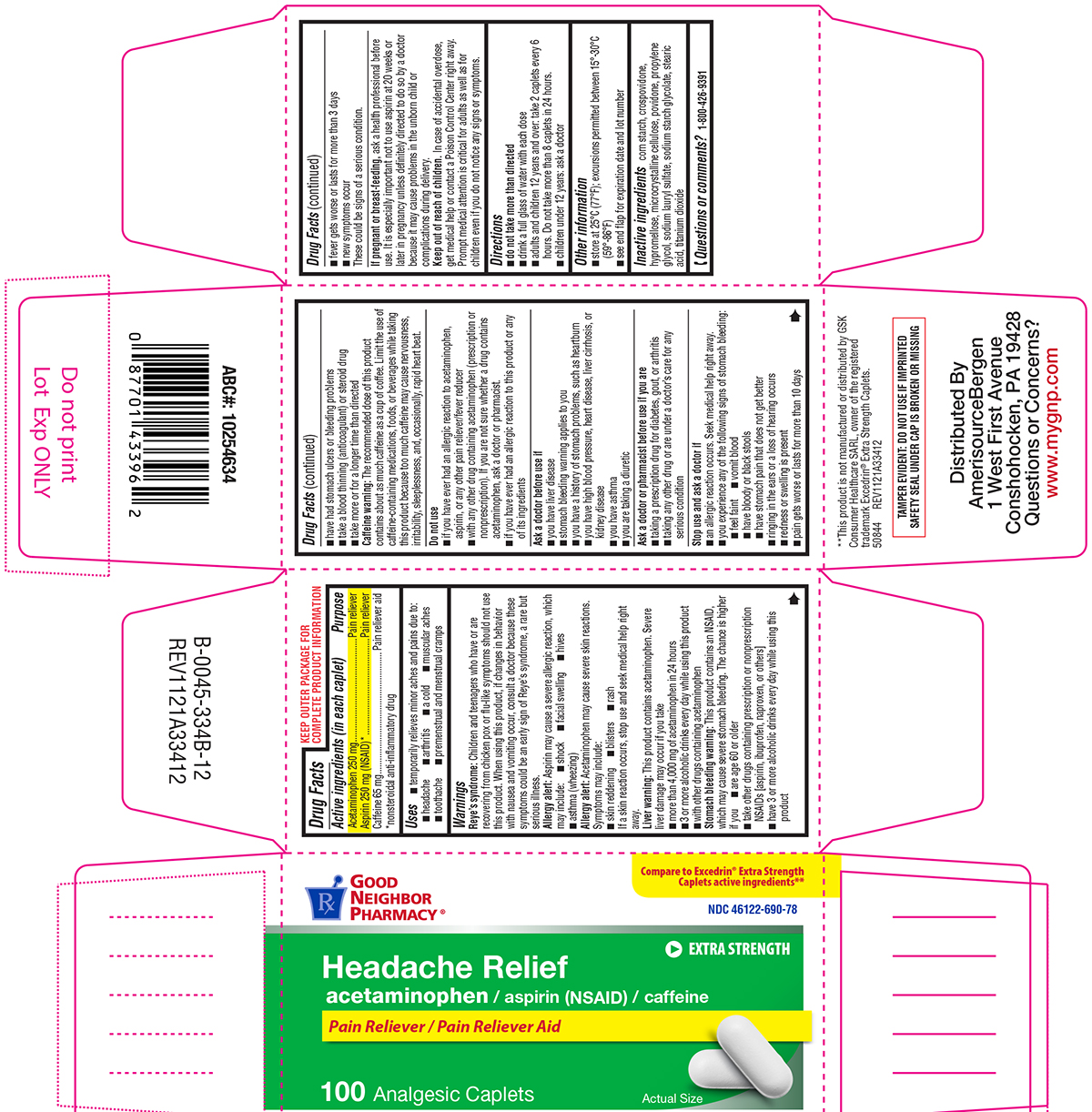
Label: HEADACHE RELIEF- acetaminophen, aspirin, caffeine tablet, film coated
- NDC Code(s): 46122-690-78
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients (in each caplet)
Acetaminophen 250 mg - Aspirin 250 mg (NSAID)* Caffeine 65 mg - *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever - Pain reliever - Pain reliever aid
-
Uses
temporarily relieves minor aches and pains due to: headache - arthritis - a cold - muscular aches - toothache - premenstrual and menstrual cramps
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea ...
-
Directions
do not take more than directed - drink a full glass of water with each dose - adults and children 12 years and over: take 2 caplets every 6 hours. Do not take more than 8 caplets in 24 ...
-
Other information
store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) see end flap for expiration date and lot number
-
Inactive ingredients
corn starch, crospovidone, hypromellose, microcrystalline cellulose, povidone, propylene glycol, sodium lauryl sulfate, sodium starch glycolate, stearic acid, titanium dioxide
-
Questions or comments?
1-800-426-9391
-
Principal display panel
GOOD - NEIGHBOR - PHARMACY® Compare to Excedrin® Extra Strength - Caplets active ingredients** NDC 46122-690-78 - EXTRA STRENGTH - Headache Relief - acetaminophen / aspirin (NSAID) / caffeine - Pain ...
-
INGREDIENTS AND APPEARANCEProduct Information