Label: GAMASTAN (immune globulin- human injection, solution
GAMASTAN (immune globulin- human injection, solution
-
NDC Code(s):
13533-335-04,
13533-335-12,
13533-335-13,
13533-335-40, view more13533-635-04, 13533-635-12, 13533-635-13, 13533-635-40
- Packager: GRIFOLS USA, LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GAMASTAN safely and effectively. See full prescribing information for GAMASTAN.
GAMASTAN[immune globulin (human)], solution for intramuscular injection
Initial U.S. Approval: 1944
WARNING: THROMBOSIS
See full prescribing information for complete boxed warning
- Thrombosis may occur with immune globulin products, including GAMASTAN. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. [see Warnings and Precautions (5.2), Patient Counseling Information (17)]
-
For patients at risk of thrombosis, do not exceed the recommended dose of
GAMASTAN. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [Warnings and Precautions (5.2)]
INDICATIONS AND USAGE
GAMASTAN is a human immune globulin indicated:
- For prophylaxis following exposure to hepatitis A. (1.1)
- To prevent or modify measles in a susceptible person exposed fewer than 6 days previously. (1.2)
- To modify varicella. (1.3)
- To modify rubella in exposed women who will not consider a therapeutic abortion. (1.4)
- Not indicated for routine prophylaxis or treatment of viral hepatitis type B, rubella, poliomyelitis, mumps or varicella. (1.5)
DOSAGE AND ADMINISTRATION
For intramuscular use only. Do not administer intravenously.
Indication Dosage Instruction Hepatitis A (2.1) 0.1 mL/kg Administer within two weeks of prior exposure to hepatitis A. Administer before departure to persons traveling to areas with endemic hepatitis A: 0.1 mL/kg if the length of stay will be up to 1 month 0.2 mL/kg if the length of stay will be up to 2 months; repeat every 2 months for longer stays. Measles (2.1) 0.25 mL/kg Administer within 6 days of exposure. 0.5 mL/kg Immediately administer (maximum dose, 15 mL) to an immunocompromised child. Varicella (2.1) 0.6 mL/kg to 1.2 mL/kg Administer promptly if Varicella-Zoster Immune Globulin (Human) is unavailable. Rubella (2.1) 0.55 mL/kg Only administer to exposed pregnant women who will not consider a therapeutic abortion. DOSAGE FORMS AND STRENGTHS
GAMASTAN is a sterile, 16.5% protein solution supplied in 2 mL and 10 mL single-dose vials. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Patients with known hypersensitivity to immune globulin preparations are at greater risk of developing severe hypersensitivity and anaphylactic reactions. Have epinephrine available immediately to treat any acute severe hypersensitivity reactions. (5.1)
- Do not administer intravenously because of the potential for serious reactions (e.g., Renal Dysfunction/Failure/Hemolysis, Transfusion-Related Acute Lung Injury [TRALI]). (5.3)
- GAMASTAN is made from human blood; it may carry a risk of transmitting infectious agents, e. g, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.4)
ADVERSE REACTIONS
The most common adverse reaction reported for GAMASTAN S/D during post-approval use was fatigue. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Grifols Therapeutics LLC at 1-800-520-2807 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Defer live vaccine administration for up to 6 months. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS
1 INDICATIONS AND USAGE
1.1 Hepatitis A
1.2 Measles (Rubeola)
1.3 Varicella
1.4 Rubella
1.5 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Handling
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Thrombosis
5.3 Systemic Reactions
5.4 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: THROMBOSIS
- Thrombosis may occur with immune globulin products, including GAMASTAN. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. [see Warnings and Precautions (5.2), Patient Counseling Information (17)]
- For patients at risk of thrombosis, do not exceed the recommended dose of GAMASTAN. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [Warnings and Precautions (5.2)]
-
1 INDICATIONS AND USAGE
GAMASTAN is a human immune globulin indicated for:
1.1 Hepatitis A
GAMASTAN is indicated for prophylaxis following exposure to hepatitis A.(1,2) The prophylactic value of GAMASTAN is greatest when given before or soon after exposure to hepatitis A. GAMASTAN is not indicated in persons with clinical manifestations of hepatitis A or in those exposed more than 2 weeks previously.
1.2 Measles (Rubeola)
GAMASTAN is indicated to prevent or modify measles in a susceptible person exposed fewer than 6 days previously.(3) A susceptible person is one who has not been vaccinated and has not had measles previously.
- GAMASTAN may be especially indicated for susceptible household contacts of measles patients, particularly contacts under 1 year of age, for whom the risk of complications is highest.(3)
- GAMASTAN is also indicated for pregnant women without evidence of immunity.
- Do not give GAMASTAN and measles vaccine at the same time. If a child is older than 12 months and has received GAMASTAN, give measles vaccine about five months later when the measles antibody titer will have disappeared.(3,4) [see Drug Interactions (7)] If a susceptible child exposed to measles is immunocompromised, give GAMASTAN immediately.
1.3 Varicella
GAMASTAN is indicated to modify varicella.
- Passive immunization against varicella in immunosuppressed patients is best accomplished by use of Varicella Zoster Immune Globulin (Human). If unavailable, GAMASTAN, promptly given, may also modify varicella. (5,6)
1.4 Rubella
GAMASTAN is indicated to modify rubella in exposed women who will not consider a therapeutic abortion.
- Some studies suggest that the use of GAMASTAN in exposed, susceptible women can lessen the likelihood of infection and fetal damage; therefore, GAMASTAN may benefit those women who will not consider a therapeutic abortion.(7)
- Do not give GAMASTAN for routine prophylaxis of rubella in early pregnancy to an unexposed woman.(7)
1.5 Limitations of Use
- GAMASTAN is not standardized with respect to antibody titers against hepatitis B surface antigen (HBsAg) and must not be used for prophylaxis of viral hepatitis type B. Prophylactic treatment to prevent hepatitis B can best be accomplished with use of Hepatitis B Immune Globulin (Human), often in combination with Hepatitis B Vaccine.(8)
- GAMASTAN is not indicated for routine prophylaxis or treatment of rubella, poliomyelitis, mumps, or varicella.
- GAMASTAN may be especially indicated for susceptible household contacts of measles patients, particularly contacts under 1 year of age, for whom the risk of complications is highest.(3)
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
Do not administer intravenously.
2.1 Dose
Indication Dosage Instruction Prophylaxis for exposure to hepatitis A 0.1 mL/kg* Administer within two weeks of prior exposure for household and institutional hepatitis A case contacts. Administer before departure to persons traveling to areas with endemic hepatitis A: 0.1 mL/kg if the length of stay will be up to 1 month(9,10) 0.2 mL/kg if the length of stay will be up to 2 months(9,10) 0.2 mL/kg if the length of stay will be 2 months or longer; repeat every 2 months.(9,10) Prevent or modify measles in a susceptible person exposed fewer than six days previously 0.25 mL/kg† Administer to a susceptible person within 6 days of exposure. 0.5 mL/kg Immediately administer (maximum dose, 15 mL) to an immunocompromised child. Modify varicella 0.6 mL/ kg to 1.2 mL/kg Administer promptly only if Varicella-Zoster Immune Globulin (Human) is unavailable. Modify rubella only in an exposed woman who will not consider a therapeutic abortion 0.55 mL/kg Only administer to an exposed pregnant woman who will not consider a therapeutic abortion. 2.2 Preparation and Handling
- If the product shows any sign of tampering, do not use it and notify Grifols Therapeutics LLC immediately [1-800-520-2807].
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. GAMASTAN is a clear or slightly opalescent, and colorless to pale yellow sterile solution.
2.3 Administration
- Administer GAMASTAN intramuscularly, preferably in the anterolateral aspects of the upper thigh and the deltoid muscle of the upper arm. Do not use the gluteal region as an injection site because of the risk of injury to the sciatic nerve.(4) [see Warnings and Precautions (5.3)]
- Draw back on the plunger of the syringe before injection in order to be certain that the needle is not in a blood vessel. [see Warnings and Precautions (5.3)]
- Divide and inject doses greater than 10 mL into several muscle sites to reduce local pain and discomfort. An individual decision as to which muscle is injected must be made for each patient based on the volume of material to be administered.
- If Hepatitis A Vaccine is recommended along with GAMASTAN, administer simultaneously but at separate anatomical sites.
- If the product shows any sign of tampering, do not use it and notify Grifols Therapeutics LLC immediately [1-800-520-2807].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
GAMASTAN is contraindicated in:
- Anaphylactic or severe systemic hypersensitivity reactions to Immune Globulin (Human).(11) [see Warnings and Precautions (5.1)]
- IgA deficient patients with antibodies against IgA and a history of hypersensitivity.(11)
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Administer GAMASTAN cautiously to patients with a history of prior systemic allergic reactions following the administration of human immunoglobulin preparations.(11) Have epinephrine available for treatment of acute allergic symptoms, should they occur.
Do not perform skin tests. In most patients the intradermal injection of concentrated gamma globulin solution with its buffers causes a localized area of inflammation which can be misinterpreted as a positive allergic reaction. In actuality, this does not represent an allergy; rather, it is localized tissue irritation of a chemical nature. Misinterpretation of the results of such tests can lead the physician to withhold beneficial human immunoglobulin from a patient who is not actually allergic to this material.
5.2 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including GAMASTAN.(12-14) Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, do not exceed the recommended dose of GAMASTAN. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [see Boxed Warning, Patient Counseling Information (17)]
5.3 Systemic Reactions
Inject intramuscularly only. Do not administer GAMASTAN intravenously because of the potential for serious reactions (e.g., Renal Dysfunction/Failure/Hemolysis, Transfusion-Related Acute Lung Injury [TRALI]). Do not inject into a blood vessel. [see Dosage and Administration (2.3)]
5.4 Transmissible Infectious Agents
GAMASTAN is made from human blood and may carry a risk of transmitting infectious agents, e. g, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. GAMASTAN is purified from human plasma obtained from healthy donors. When medicinal biological products are administered, infectious diseases due to transmission of pathogens cannot be totally excluded. However, in the case of products prepared from human plasma, the risk of transmission of pathogens is reduced by: (1) epidemiological controls on the donor population and selection of individual donors by a medical interview and screening of individual donations and plasma pools for viral infection markers; (2) testing of plasma for hepatitis C virus (HCV), human immunodeficiency virus (HIV), hepatitis B virus (HBV), HAV, and human parvovirus (B19V) genomic material; and (3) manufacturing procedures with demonstrated capacity to inactivate/remove pathogens.
No cases of transmission of viral diseases, vCJD, or CJD have ever been identified for products manufactured with the same core manufacturing process as GAMASTAN. ALL infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
-
6 ADVERSE REACTIONS
The most common adverse reaction reported for GAMASTAN S/D during post-approval use was fatigue.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use with GAMASTAN made using the previous manufacturing process, GAMASTAN S/D. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Among patients treated with GAMASTAN S/D, cases of allergic/hypersensitivity reactions including anaphylaxis have been reported. Anaphylactic reactions, although rare, have been reported following the injection of human immune globulin preparations.(11) Anaphylaxis was more likely to occur if GAMASTAN S/D was given intravenously; therefore, GAMASTAN S/D and GAMASTAN must be administered only intramuscularly.
The following have been identified as the most frequently reported post-marketing adverse reactions.
- *
- These reactions have been manifested by rash, flushing, and dyspnea
Immune system disorders Anaphylactic reaction*,
hypersensitivity*Nervous system disorders Headache Gastrointestinal disorders Nausea General disorders and administration site conditions Injection site pain, injection site inflammation, fatigue, pyrexia - 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with GAMASTAN use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with GAMASTAN. It is not known whether GAMASTAN can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of GAMASTAN in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GAMASTAN and any potential adverse effects on the breastfed infant from GAMASTAN or from the underlying maternal condition.
-
11 DESCRIPTION
GAMASTAN is a clear or slightly opalescent, and colorless or pale yellow sterile solution of polyvalent human immune globulin for intramuscular administration. GAMASTAN contains no preservative. GAMASTAN is prepared from pools of human plasma collected from healthy donors by a combination of cold ethanol fractionation, caprylate precipitation and filtration, caprylate incubation, anion-exchange chromatography, nanofiltration and low pH incubation. GAMASTAN consists of 15% to18% protein at pH of 4.1 to 4.8 in 0.16 to 0.26 M glycine.
When medicinal biological products are administered, infectious diseases due to transmission of pathogens cannot be totally excluded. However, in the case of products prepared from human plasma, the risk of transmission of pathogens is reduced by epidemiological surveillance of the donor population and selection of individual donors by medical interview; testing of individual donations and plasma pools; and the presence in the manufacturing processes of steps with demonstrated capacity to inactivate/remove pathogens.
In the manufacturing process of GAMASTAN, there are several steps with the capacity for viral inactivation or removal. The main steps of the manufacturing process that contribute to the virus clearance capacity are as follows:
- Caprylate precipitation/depth filtration
- Caprylate incubation
- Depth filtration
- Column chromatography
- Nanofiltration
- Low pH final container incubation
To provide additional assurance of the pathogen safety of the final product, the capacity of the GAMASTAN manufacturing process to remove and/or inactivate viruses has been demonstrated by laboratory spiking studies on a scaled down process model using a wide range of viruses with diverse physicochemical properties.
The combination of all of the above mentioned measures provides the final product with a high margin of safety from the potential risk of transmission of infectious viruses.
The caprylate/chromatography manufacturing process was also investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the variant Creutzfeldt-Jakob disease (vCJD), and Creutzfeldt-Jakob disease (CJD) agents.(15) These studies provide reasonable assurance that low levels of vCJD/CJD agent infectivity, if present in the starting material, would be removed by the caprylate/chromatography manufacturing process.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The polyclonal antibody in GAMASTAN is a passive immunizing agent to neutralize viruses, such as hepatitis A and measles viruses, to prevent or ameliorate disease.
12.2 Pharmacodynamics
The prophylactic value of GAMASTAN is greatest when given before or soon after exposure.
12.3 Pharmacokinetics
Peak levels of immunoglobulin G are obtained approximately two days after intramuscular injection of GAMASTAN.(16) The half-life of IgG in the circulation of individuals with normal IgG levels is 23 days.(17)
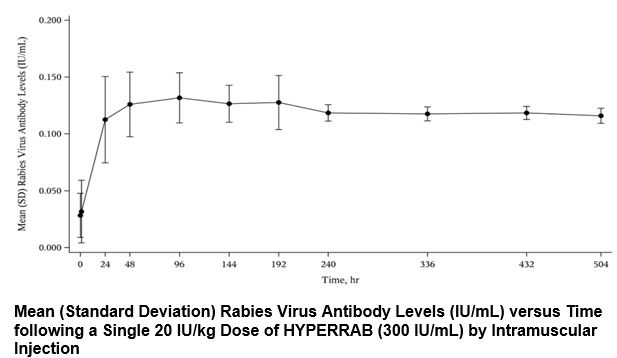
In a clinical study, 12 healthy human subjects received a 20 IU/kg intramuscular dose of HYPERRAB [rabies immune globulin (human)] made using the same manufacturing process as GAMASTAN. Detectable passive rabies neutralizing antibody was present by 24 hours and persisted through the 21 day follow-up evaluation period. The figure below shows the mean levels of rabies virus antibodies in IU/mL across the 21 day evaluation period and indicates that the titer remains stable during this period.
-
15 REFERENCES
- Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2006;55(RR-07):1-23.
- Centers for Disease Control and Prevention. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2007;56(41);1080-4.
- Centers for Disease Control and Prevention. Measles, mumps, and rubella - Vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1998;47(RR-08):1-57.
- Centers for Disease Control and Prevention. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2011;60(RR02):1-61.
- Centers for Disease Control and Prevention. Prevention of varicella. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2007;56(RR04):1-40.
- Gershon AA, Piomelli S, Karpatkin M, et al. Antibody to varicella-zoster virus after passive immunization against chickenpox. J Clin Microbiol. 1978;8(6): 733-5.
- Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2013;62(RR-04):17-8.
- Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II. Immunization of adults. MMWR. 2006;55(RR-16):1-33.
- Zaaijer HL, Leentvaar-Kuijpers A, Rotman H, et al. Hepatitis A antibody titres after infection and immunization: implications for passive and active immunization. J Med Virol. 1993;40:22-7.
- Tejada-Strop A, Costafreda MI, Dimitrova Z, et al. Evaluation of potencies of immune globulin products against hepatitis A. JAMA Intern. Med. 2017;177(3):430-2.
- Fudenberg HH. Sensitization to immunoglobulins and hazards of gamma globulin therapy. In: Merler E, editor. Immunoglobulins: biologic aspects and clinical uses. Washington DC: National Academy of Sciences; 1970, p. 211-20.
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology. 1994;44:223-6.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-8.
- Wolberg AS, Kon RH, Monroe DM, et al. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am J Hematol. 2000;65,30-4.
- Barnette D, Roth NJ, Hotta J, et al. Pathogen safety profile of a 10% IgG preparation manufactured using a depth filtration-modified process. Biologicals. 2012;40:247-53.
- Smith GN, Griffiths B, Mollison D, et al. Uptake of IgG after intramuscular and subcutaneous injection. Lancet. 1972;1(7762): 1208-12.
- Waldmann TA, Strober W, Blaese RM. Variations in the metabolism of immunoglobulins measured by turnover rates. In: Merler E, editor. Immunoglobulins: biologic aspects and clinical uses. Washington, DC: National Academy of Sciences; 1970, p. 33-51.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient.
- Instruct the patient to immediately report symptoms of thrombosis. These symptoms may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body. [see Warnings and Precautions (5.2)]
- Inform the patient that GAMASTAN is made from human plasma and may carry a risk of transmitting infectious agents that can cause disease. While the risk that GAMASTAN can transmit an infectious agent has been reduced by screening plasma donors for prior exposure, testing donated plasma, and including manufacturing steps with the capacity to inactivate and/ or remove pathogens, instruct the patient to report any symptoms that concern them. [see Boxed Warning,Warnings and Precautions (5.4)]
- Inform the patient that GAMASTAN can interfere with their immune response to live virus vaccines such as measles, mumps and rubella. Inform patients to notify their healthcare professional of this potential interaction when they are receiving vaccinations. [see Drug Interactions (7)]
Manufactured by:

Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3063873
-
PRINCIPAL DISPLAY PANEL
LEFT PANEL
NDC 13533-335-12 10 mL
Immune
Globulin
(Human)
GamaSTAN®
10 mL
Solution for
Intramuscular Injection
Rx only
FOR INTRAMUSCULAR
INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY.
GRIFOLS
TOP FLAP
NDC 13533-335-12
GTIN 00313533335120
LOT XXXXXXXXXX
EXP DDMMMYY
SN XXXXXXXXXXXXXXXX
GamaSTAN® 10 mL
SECOND PANEL
Immune Globulin (Human) is a
sterile solution of immuno-
globulin containing 15%-18%
protein stabilized with 0.16 to
0.26 M glycine.
No U.S. standard of potency for
viral hepatitis antibodies.
10 mL
Not made with natural rubber
latex.
No preservative
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
GRIFOLS
THIRD PANEL
NDC 13533-335-12 10 mL
Immune
Globulin
(Human)
GamaSTAN®
10 mL
Solution for
Intramuscular Injection
Rx only
FOR INTRAMUSCULAR
INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY.
GRIFOLS
Bottom Tab
Carton: 3060459
RIGHT PANEL
The patient and physician
should discuss the risks and
benefits of this product.
One Single-dose Vial
For complete dosage and
administration information,
read enclosed package insert.
Store at 2-8°C (36-46°F).
Do not freeze.
10 mL
Discard unused portion.
If the shrink band is absent or
shows any sign of tampering,
do not use the product and
notify Grifols Therapeutics LLC
immediately.
Not returnable for credit or
exchange.
GRIFOLS
NDC 13533-335-13 10 mL
Immune Globulin (Human)
GamaSTAN®
The patient and physician should discuss
the risks and benefits of this product.
One Single-dose Vial
Do not give intravenously.
Dosage: Read package insert.
Grifols Therapeutics LLC
RTP, NC 27709 USA
Rx only
U.S. License No. 1871
3060451
Lot/Exp.

-
INGREDIENTS AND APPEARANCE
GAMASTAN
immune globulin (human) injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:13533-335 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Immunoglobulin G (UNII: 66Y330CJHS) (Human Immunoglobulin G - UNII:66Y330CJHS) Human Immunoglobulin G 0.165 g in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (colorless or pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13533-335-04 1 in 1 CARTON 1 NDC:13533-335-40 2 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:13533-335-12 1 in 1 CARTON 2 NDC:13533-335-13 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101134 02/05/2018 GAMASTAN
immune globulin (human) injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:13533-635 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Immunoglobulin G (UNII: 66Y330CJHS) (Human Immunoglobulin G - UNII:66Y330CJHS) Human Immunoglobulin G 0.165 g in 1 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (Clear liquid, colorless to pale yellow or pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13533-635-04 1 in 1 CARTON 1 NDC:13533-635-40 2 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:13533-635-12 1 in 1 CARTON 2 NDC:13533-635-13 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101134 08/14/1996 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations Grifols Therapeutics LLC 611019113 manufacture(13533-335, 13533-635)


