Label: PRAVASTATIN SODIUM tablet
- NDC Code(s): 50090-0973-0, 50090-0973-1
- Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 0093-7201
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRAVASTATIN SODIUM TABLETS safely and effectively. See full prescribing information for PRAVASTATIN SODIUM TABLETS. PRAVASTATIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPravastatin sodium tablets are indicated: To reduce the risk of myocardial infarction, myocardial revascularization procedures, and cardiovascular mortality in adults with elevated low-density ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage - and Administration Information - Take pravastatin sodium tablets orally once daily as a single dose at any time of the day, with or without food. For patients that require a ...
-
3 DOSAGE FORMS AND STRENGTHSPravastatin sodium tablets, USP are supplied as: 10 mg of pravastatin sodium: Pink, unscored, round tablet, debossed “TEVA” on one side and “771” on the other side. 20 mg of pravastatin sodium ...
-
4 CONTRAINDICATIONSAcute liver failure or decompensated cirrhosis [see Warnings and Precautions (5.3)]. Hypersensitivity to any pravastatin or any excipients in pravastatin sodium tablets.
-
5 WARNINGS AND PRECAUTIONS5.1 Myopathy and - Rhabdomyolysis - Pravastatin may cause myopathy and rhabdomyolysis. Acute kidney injury secondary to myoglobinuria and rare fatalities have occurred as a result of rhabdomyolysis ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described below and elsewhere in the labeling: Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)] Immune-Mediated Necrotizing Myopathy ...
-
7 DRUG INTERACTIONS7.1 Drug Interactions - that Increase the Risk of Myopathy and Rhabdomyolysis with Pravastatin - Pravastatin is a substrate of the transport protein OATP1B1. Pravastatin plasma levels can be ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Discontinue pravastatin when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient. Pravastatin decreases ...
-
10 OVERDOSAGENo specific antidotes for pravastatin are known. Contact Poison Control (1-800-222-1222) for latest recommendations.
-
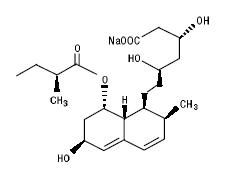
11 DESCRIPTIONPravastatin sodium, USP is a statin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Pravastatin sodium, USP is designated chemically as 1-Naphthalene-heptanoic acid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pravastatin is a reversible inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts HMG-CoA to mevalonate, a precursor of cholesterol. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year study in rats fed pravastatin at doses of 10, 30, or 100 mg/kg body weight, there was an increased incidence of ...
-
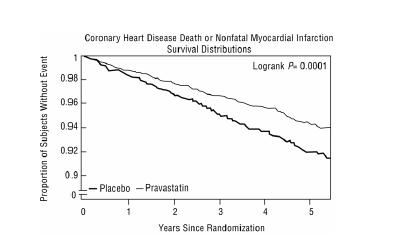
14 CLINICAL STUDIESPrevention of Coronary Heart Disease - In the Pravastatin Primary Prevention Study (WOS), the effect of pravastatin on fatal and nonfatal CHD was assessed in 6,595 male patients 45 to 64 years of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGProduct: 50090-0973 - NDC: 50090-0973-0 30 TABLET in a BOTTLE - NDC: 50090-0973-1 90 TABLET in a BOTTLE
-
17 PATIENT COUNSELING INFORMATIONMyopathy and Rhabdomyolysis - Advise patients that pravastatin sodium tablets may cause myopathy and rhabdomyolysis. Inform patients that the risk is increased when taking certain types of ...
-
Pravastatin Sodium

-
INGREDIENTS AND APPEARANCEProduct Information