Label: MECLIZINE HYDROCHLORIDE- meclizine tablet
- NDC Code(s): 50090-7417-0, 50090-7417-1, 50090-7417-4, 50090-7417-8
- Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 59651-808
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MECLIZINE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for MECLIZINE HYDROCHLORIDE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMeclizine hydrochloride tablets are indicated for the treatment of vertigo associated with diseases affecting the vestibular system in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage is 25 mg to 100 mg daily administered orally, in divided doses, depending upon clinical response. 2.2 Administration Instructions - Tablets ...
-
3 DOSAGE FORMS AND STRENGTHSMeclizine Hydrochloride Tablets, USP are available containing 12.5 mg, 25 mg or 50 mg of meclizine dihydrochloride equivalent to 10.53 mg, 21.07 mg or 42.14 mg of meclizine free base ...
-
4 CONTRAINDICATIONSMeclizine hydrochloride tablets are contraindicated in patients with a hypersensitivity to meclizine or any of the inactive ingredients [see Adverse Reactions (6) and Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Drowsiness - Since drowsiness may occur with use of meclizine hydrochloride tablets, patients should be warned of this possibility and cautioned against driving a car or operating dangerous ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of meclizine hydrochloride were identified in clinical studies or postmarketing reports. Because some of these reactions were reported ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - There may be increased CNS depression when meclizine hydrochloride tablets are administered concurrently with other CNS depressants, including alcohol [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary: Data from epidemiological studies have not generally indicated a drug-associated risk of major birth defects with meclizine during pregnancy. However, in a ...
-
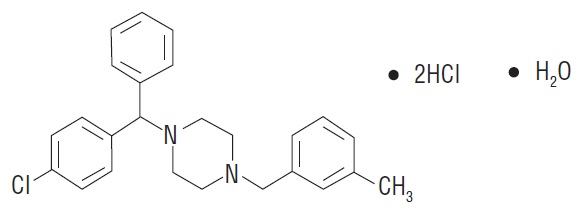
11 DESCRIPTIONMeclizine hydrochloride, USP a histamine (H1) receptor antagonist, is a white to slight yellowish crystalline powder. It has the following structural formula: Chemically, meclizine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which meclizine exerts its therapeutic effect is unknown but is presumed to involve antagonism of the histamine H1 receptor. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Animal studies to assess the carcinogenic potential of meclizine have not been conducted. Mutagenesis: Genetic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGProduct: 50090-7417 - NDC: 50090-7417-0 20 TABLET in a BOTTLE - NDC: 50090-7417-1 30 TABLET in a BOTTLE - NDC: 50090-7417-4 10 TABLET in a BOTTLE - NDC: 50090-7417-8 90 TABLET in a ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions: Advise patients that the tablets must be swallowed whole, but chewable tablets must be chewed or crushed completely before swallowing [see Dosage and Administration ...
-
MECLIZINE HYDROCHLORIDE

-
INGREDIENTS AND APPEARANCEProduct Information