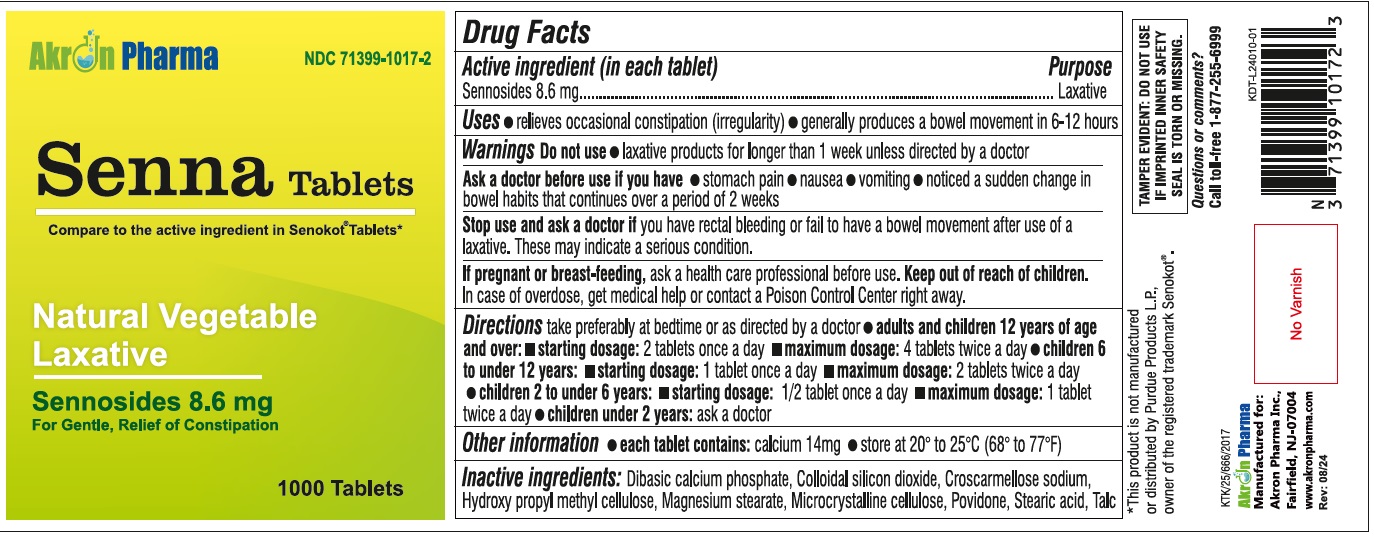
Label: SENNA- sennosides tablet
- NDC Code(s): 71399-1017-1, 71399-1017-2, 71399-1017-3
- Packager: Akron Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredient (in each tablet) Sennosides 8.6 mg
-
Purpose
Laxative
-
Uses
relieves occasional constipation (irregularity) generally causes a bowel movement in 6-12 hours
-
WARNINGSDo not use - laxative products for longer than one week unless directed by a doctor - Do not use - laxative products for longer than one week unless directed by a doctor - Ask a doctor before ...
-
Directions
take preferably at bedtime or as directed by a doctor - agestarting dosagemaximum dosage - Adults and children - 12 years of age and older - 2 tablets - once a day - 4 tablets - twice a day - Children ...
-
Other information
Each tablet contains: Calcium 14 mg - Store between 15º to 30ºC (59 to 86F)
-
Inactive ingredients
microcrystaline cellulose, dicalcium phosphate, sodium starch glycolate, croscarmellose sodium, hypromellose, povidone, collodial silicon dioxide, megnesium stearate.
-
Questions or comments?
1-(877) 255-6999
-
SPL UNCLASSIFIED SECTIONTamper Evident: Do not use if imprinted inner safety seal is torn or missing. This product is not manufactured or distributed by Purdue Products L.P., Owner of the registered trademark Senokot ...
-
PRINCIPAL DISPLAY PANEL
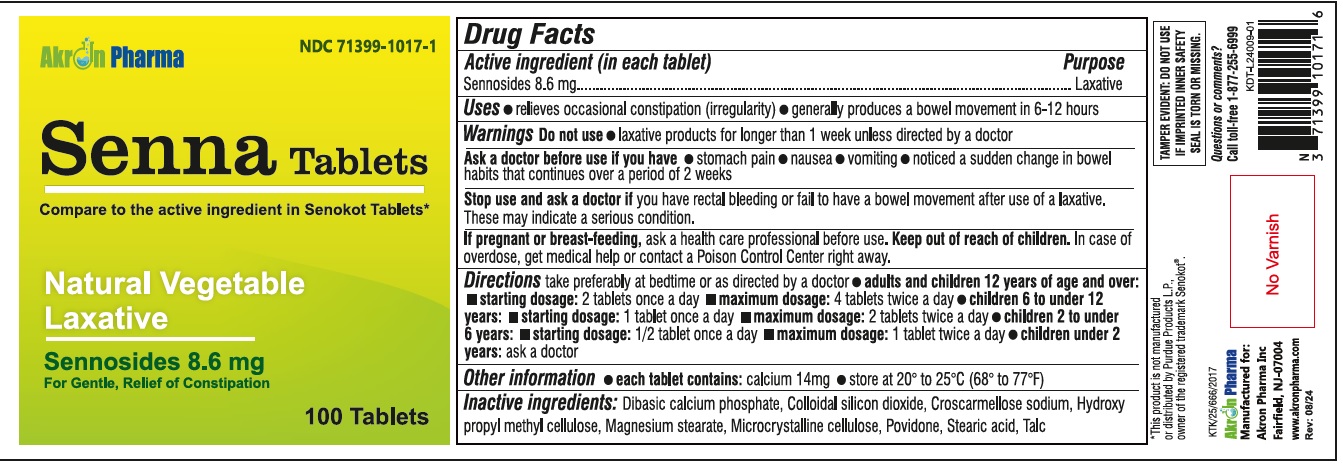 ...
... -
INGREDIENTS AND APPEARANCEProduct Information