Label: PRECEDEX- dexmedetomidine hydrochloride injection, solution
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...view full title
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...
- NDC Code(s): 0409-1434-01, 0409-1596-01, 0409-1596-10, 0409-1638-02, view more
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRECEDEX safely and effectively. See full prescribing information for PRECEDEX. PRECEDEX™ (dexmedetomidine hydrochloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Intensive Care Unit Sedation - PRECEDEX is indicated for sedation of initially intubated and mechanically ventilated adult patients during treatment in an intensive care setting. PRECEDEX ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration Instructions - • PRECEDEX dosing should be individualized and titrated to desired clinical response. • PRECEDEX is not indicated for infusions lasting longer than 24 ...
-
3 DOSAGE FORMS AND STRENGTHSPRECEDEX Presentations Requiring Dilution: PRECEDEX (dexmedetomidine hydrochloride) injection is a clear and colorless solution, to be used after dilution. It is available as: • 200 mcg/2 mL ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Drug Administration - PRECEDEX should be administered only by persons skilled in the management of patients in the intensive care or operating room setting. Due to the known pharmacological ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Hypotension, bradycardia and sinus arrest [see Warnings and Precautions (5.2)] • Transient ...
-
7 DRUG INTERACTIONS7.1 Anesthetics, Sedatives, Hypnotics, Opioids - Co-administration of PRECEDEX with anesthetics, sedatives, hypnotics, and opioids is likely to lead to an enhancement of effects. Specific studies ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published randomized controlled trials and case reports over several decades of use with intravenously administered dexmedetomidine during ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - PRECEDEX (dexmedetomidine hydrochloride) is not a controlled substance. 9.3 Dependence - The dependence potential of PRECEDEX has not been studied in humans. However ...
-
10 OVERDOSAGEThe tolerability of PRECEDEX was studied in one study in which healthy adult subjects were administered doses at and above the recommended dose of 0.2 mcg/kg/hr to 0.7 mcg/kg/hr. The maximum blood ...
-
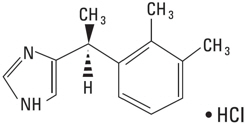
11 DESCRIPTIONPRECEDEX (dexmedetomidine hydrochloride) injection (100 mcg/mL) is a sterile, nonpyrogenic solution suitable for intravenous infusion following dilution. PRECEDEX (dexmedetomidine hydrochloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - PRECEDEX is a relatively selective centrally acting alpha2-adrenergic agonist with sedative properties. Alpha2 selectivity is observed in animals following slow ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal carcinogenicity studies have not been performed with dexmedetomidine. Mutagenesis - Dexmedetomidine was ...
-
14 CLINICAL STUDIESThe safety and efficacy of PRECEDEX has been evaluated in four randomized, double-blind, placebo-controlled multicenter clinical trials in 1,185 adult patients. 14.1 Intensive Care Unit ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGStore at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.] Do not use if product is discolored or if precipitate ...
-
17 PATIENT COUNSELING INFORMATIONPRECEDEX is indicated for short-term intravenous sedation. Dosage must be individualized and titrated to the desired clinical effect. Blood pressure, heart rate and oxygen levels will be monitored ...
-
SPL UNCLASSIFIED SECTIONDistributed by Hospira, Inc. Lake Forest, IL 60045 - LAB-1346-6.0
-
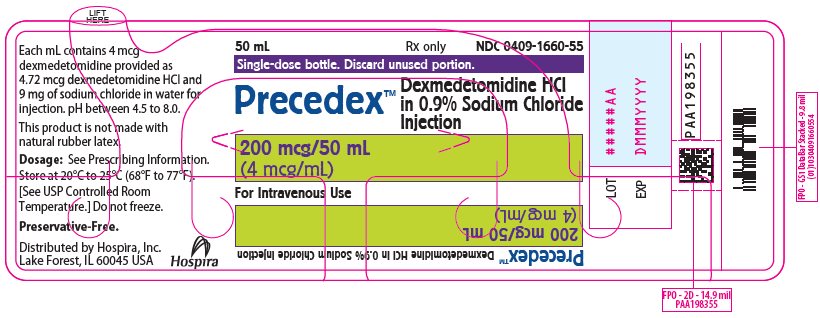
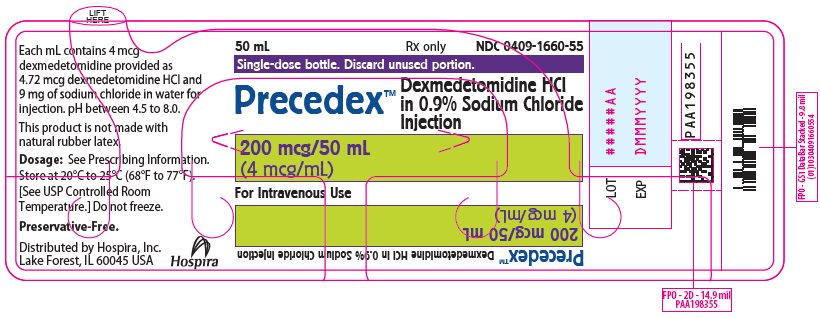
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Label50 mL - Rx only - NDC 0409-1660-55 - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 200 mcg/50 mL - (4 mcg/mL) For Intravenous Use
-
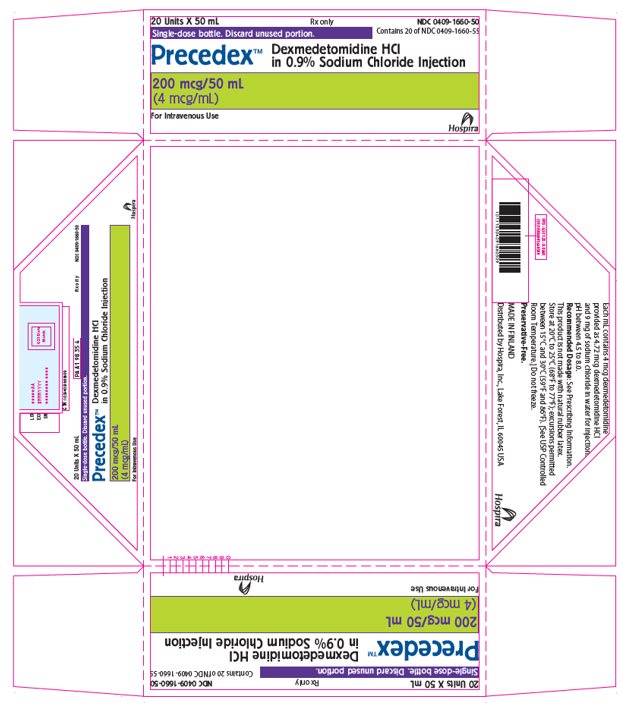
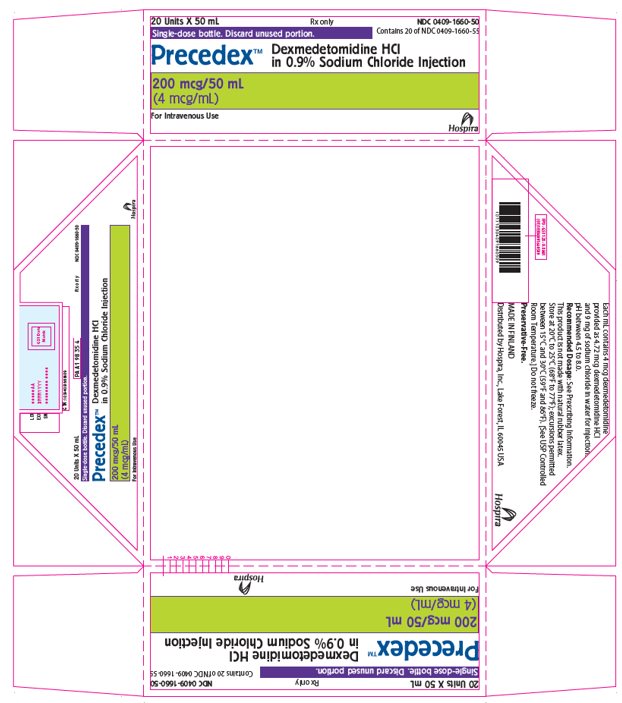
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Tray20 Units X 50 mL - Rx only - NDC 0409-1660-50 - Contains 20 of NDC 0409-1660-55 - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium Chloride Injection - 200 mcg/50 ...
-
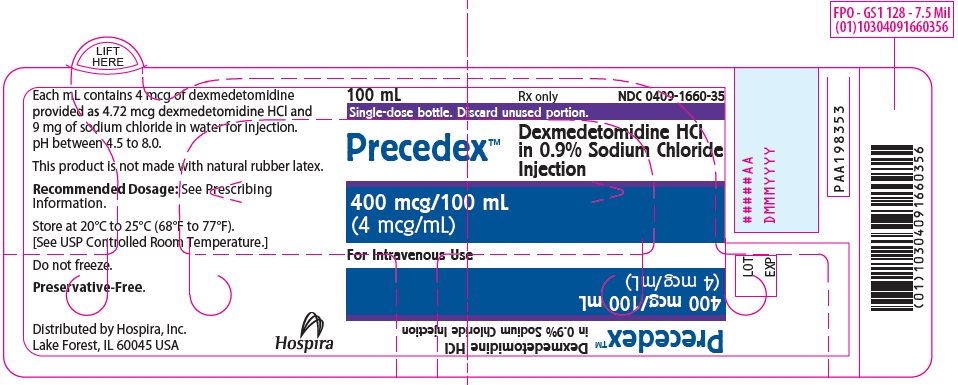
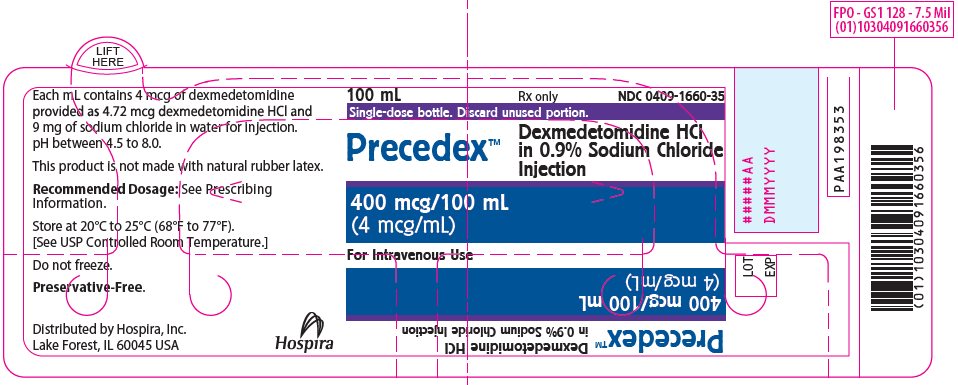
PRINCIPAL DISPLAY PANEL - 100 mL Bottle Label100 mL - Rx only - NDC 0409-1660-35 - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 400 mcg/100 mL - (4 mcg/mL) For Intravenous Use
-
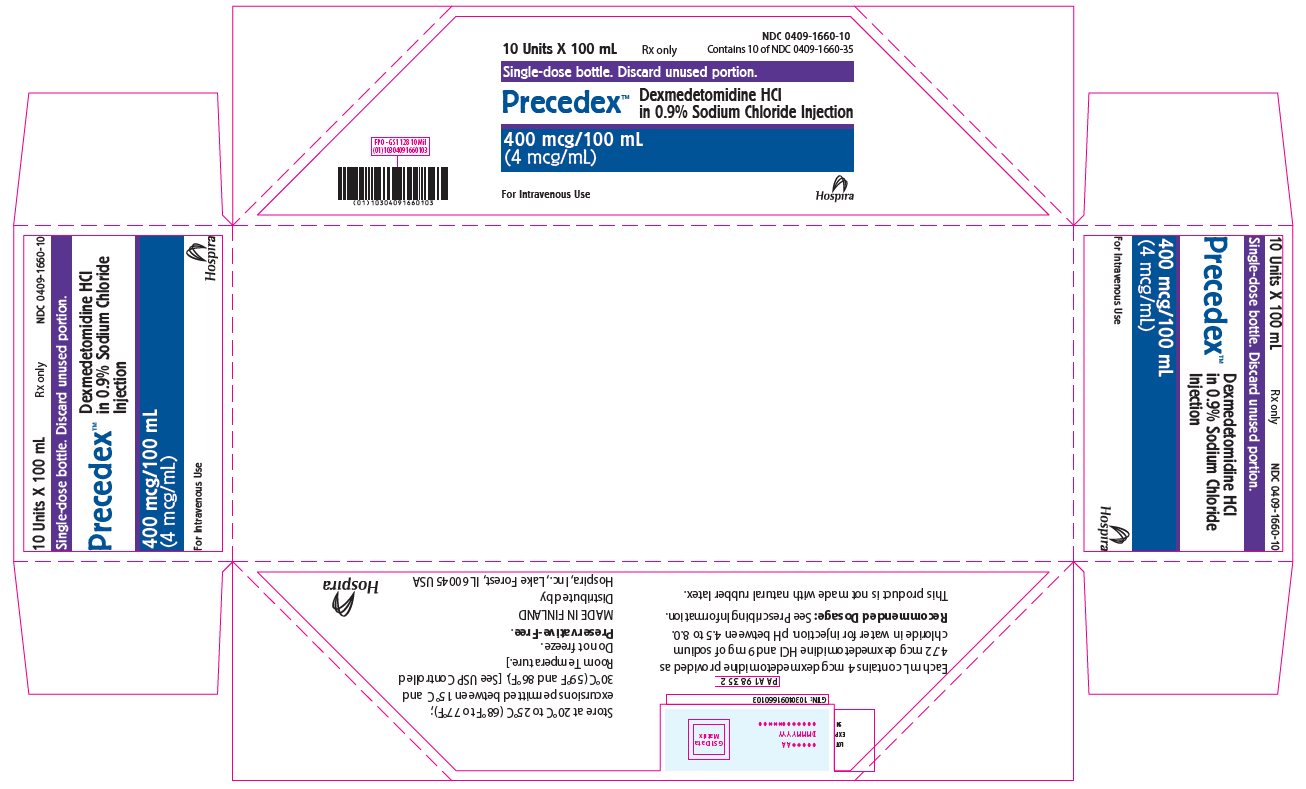
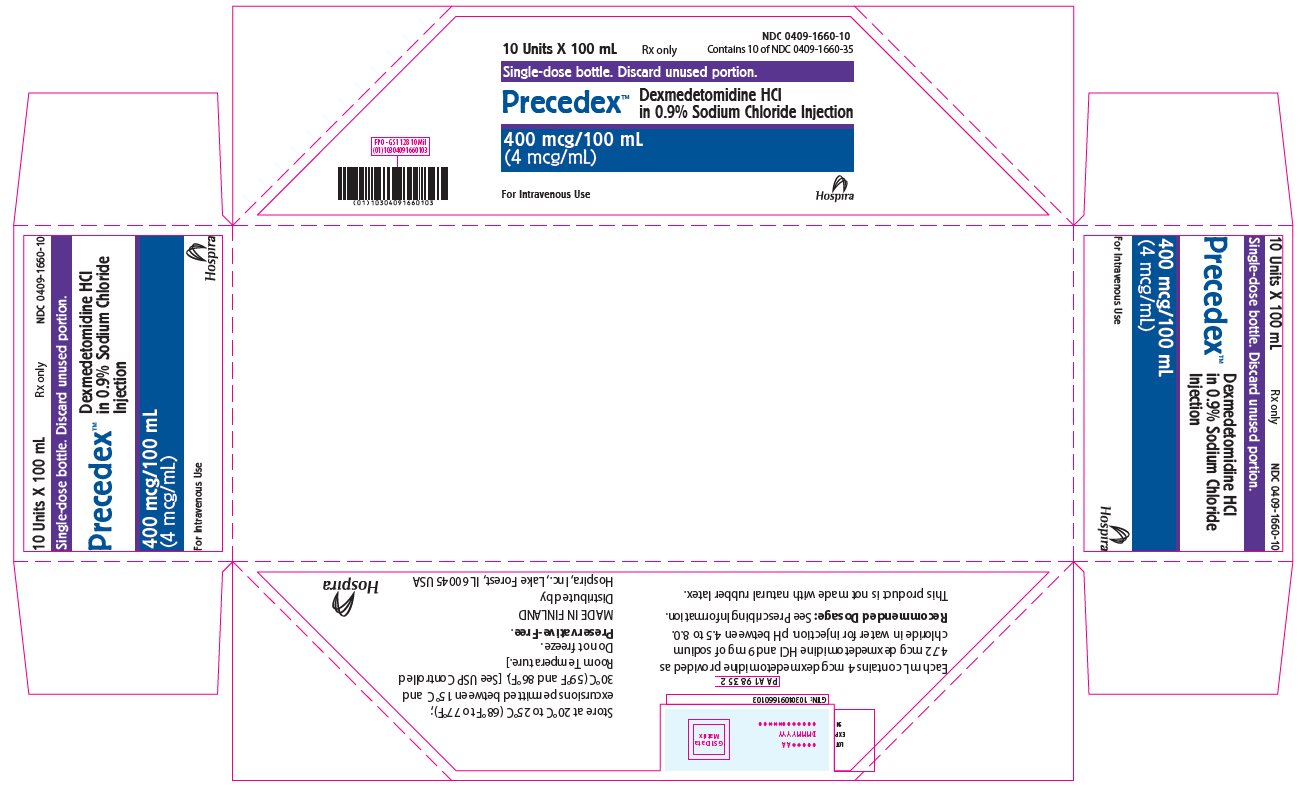
PRINCIPAL DISPLAY PANEL - 100 mL Bottle Tray10 Units X 100 mL - Rx only - NDC 0409-1660-10 - Contains 10 of NDC 0409-1660-35 - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl in 0.9% Sodium Chloride Injection - 400 mcg/100 ...
-
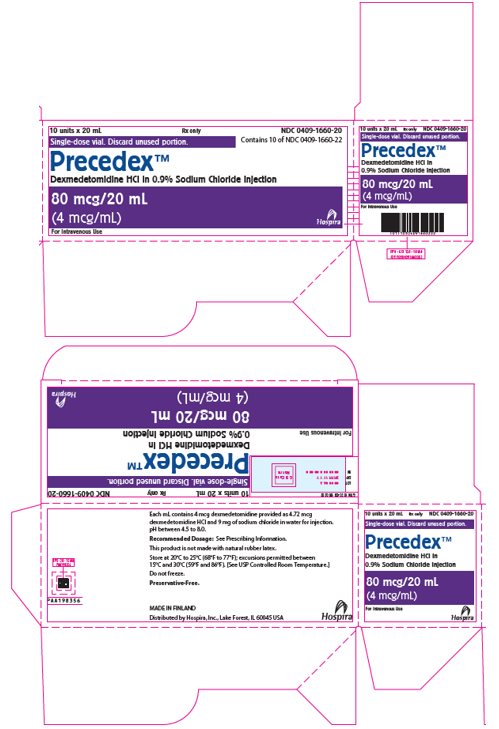
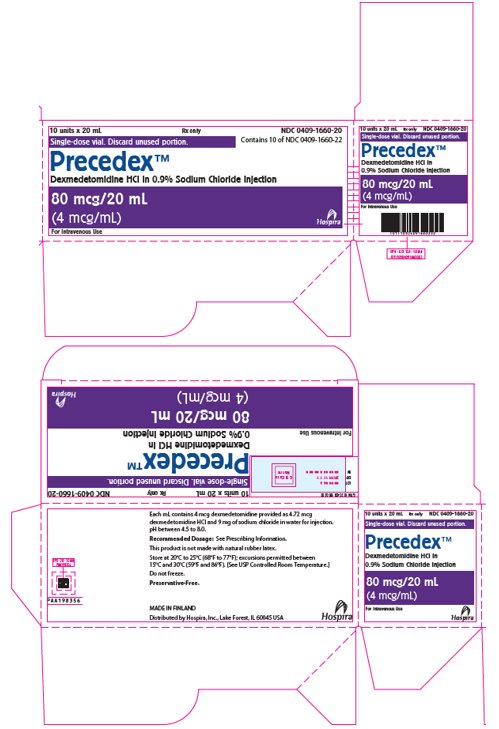
PRINCIPAL DISPLAY PANEL - 20 mL Vial Label20 mL - NDC 0409-1660-22 - Single-dose vial. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium Chloride Injection - 80 mcg/20 mL - (4 mcg/mL) Distributed by Hospira, Inc., Lake Forest ...
-
PRINCIPAL DISPLAY PANEL - 20 mL Vial Carton10 units x 20 mL - Rx only - NDC 0409-1660-20 - Contains 10 of NDC 0409-1660-22 - Single-dose vial. Discard unused portion. Precedex™ Dexmedetomidine HCl in 0.9% Sodium Chloride Injection - 80 mcg/20 ...
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial Label2 mL NDC 0409-1638-32 - Precedex™ DEXMEDETOMIDINE - HCl INJECTION - Rx only - 200 mcg/2 mL - (100 mcg/mL) For Intravenous use. MUST BE DILUTED - Dist. by Hospira, Inc., Lake Forest, IL 60045 ...
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial Tray2 mL Single-dose Fliptop Vials - Preservative-Free - NDC 0409-1638-02 - Contains 25 of NDC 0409-1638-32 - Precedex™ DEXMEDETOMIDINE HCl INJECTION - Rx only - 200 mcg/2 mL - (100 mcg/mL) For Intravenous ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bottle Label250 mL - Rx only - NDC 0409-1434-01 - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium - Chloride Injection - 1,000 mcg/250 mL - (4 mcg/mL) For Intravenous Use
-
PRINCIPAL DISPLAY PANEL - 250 mL Bottle CartonNDC 0409-1434-01 - 1 Unit X 250 mL - Rx only - Single-dose bottle. Discard unused portion. Precedex™ Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 1,000 mcg/250 mL - (4 mcg/mL) For Intravenous ...
-
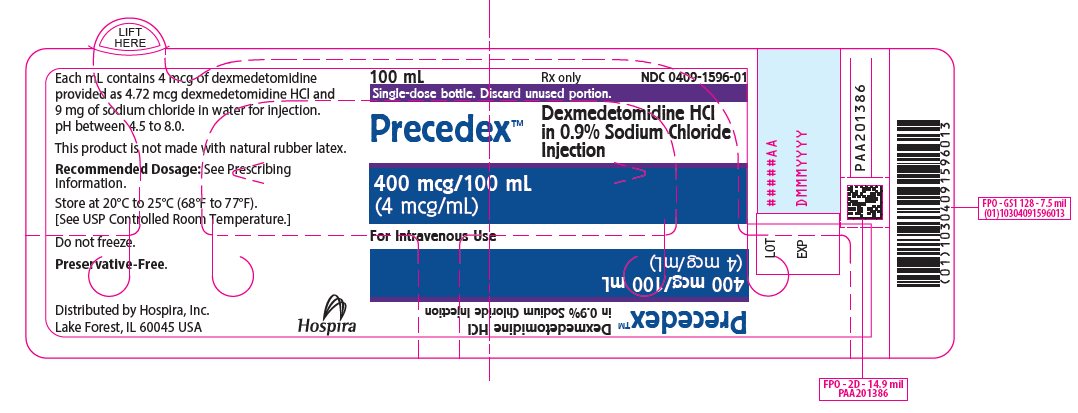
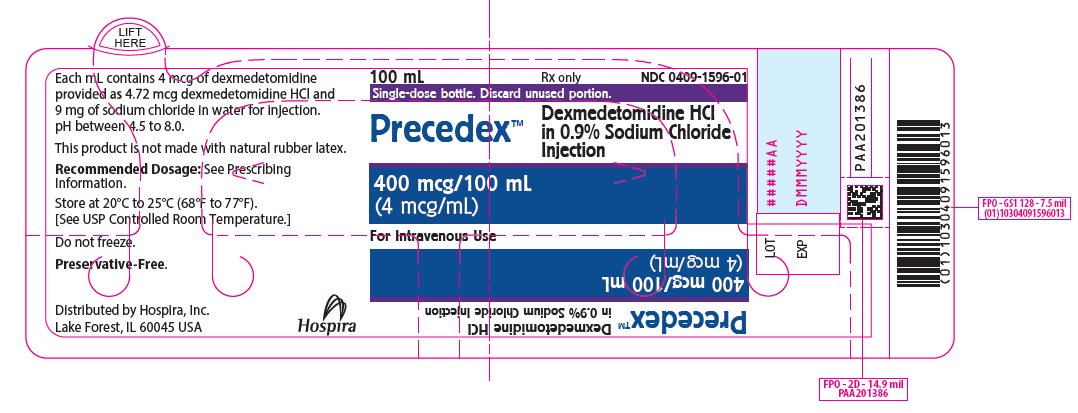
PRINCIPAL DISPLAY PANEL – 100 mL Bottle Label - 1596 100 mL - Rx only - NDC 0409-1596-01 - Single-dose bottle. Discard unused portion. PrecedexTM - Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 400 mcg/100 mL - (4 mcg/mL) For Intravenous Use
-
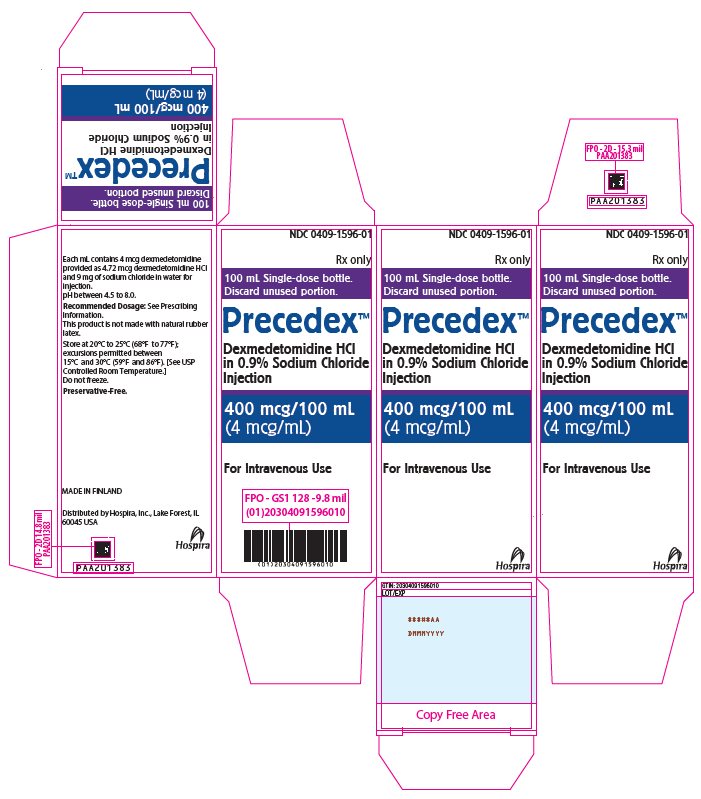
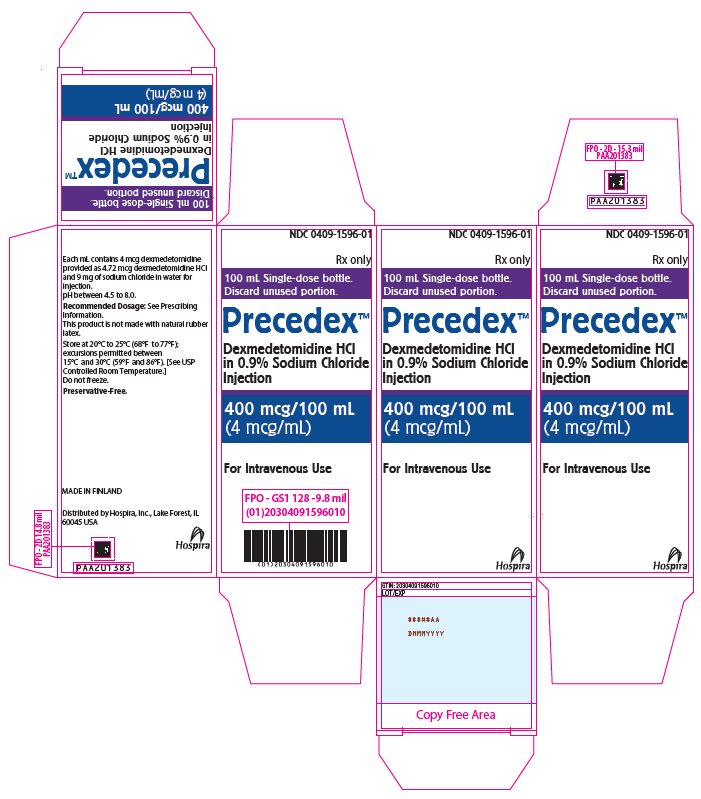
PRINCIPAL DISPLAY PANEL – 100 mL Bottle Carton - 1596 NDC 0409-1596-01 - Rx only - 100 mL Single-dose bottle. Discard unused portion. PrecedexTM Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 400 mcg/100 mL - (4 mcg/mL) For Intravenous ...
-
PRINCIPAL DISPLAY PANEL – 50 mL Bag - 7838 50 mL Single-dose. Discard unused portion. NDC 0409-7838-01 - PrecedexTM - Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - Rx only - 200 mcg/50 mL (4mcg/mL) For Intravenous Infusion ...
-
PRINCIPAL DISPLAY PANEL – 100 mL Bag - 7853 100 mL Single-dose. Discard unused portion. NDC 0409-7853-01 - PrecedexTM Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - Rx only - 400 mcg/100 mL (4 mcg/mL) For Intravenous ...
-
PRINCIPAL DISPLAY PANEL – 250 mL Bag - 7875 250 mL Single-dose. Discard unused portion. NDC 0409-7875-01 - PrecedexTM - Dexmedetomidine HCl - in 0.9% Sodium Chloride - Injection - 1,000 mcg/250 mL (4 mcg/mL) For Intravenous Infusion Each mL ...
-
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
PRECEDEX- dexmedetomidine hydrochloride injection, solution
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...view full title
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...
Number of versions: 33
RxNorm
PRECEDEX- dexmedetomidine hydrochloride injection, solution
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...view full title
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...
Get Label RSS Feed for this Drug
PRECEDEX- dexmedetomidine hydrochloride injection, solution
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...view full title
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...
NDC Codes
PRECEDEX- dexmedetomidine hydrochloride injection, solution
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...view full title
PRECEDEX- dexmedetomidine hydrochloride injection, solution, ...