Label: SENNA- sennosides capsule, gelatin coated
- NDC Code(s): 70000-0441-1
- Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each softgel)
- Purpose
- Uses
- WARNINGS
- Do not use
- Ask a doctor before use if you have
- STOP USE
- PREGNANCY OR BREAST FEEDING
-
Directions
■ take preferably at bedtime or as directed by a doctor
Age Starting Dosage Maximum Dosage adults and children 12 years of age or over 2 softgels once a day 4 softgels twice a day children 6 to under 12 years 1 softgel once a day 2 softgels twice a day children under 6 years of age ask a doctor ask a doctor - SPL UNCLASSIFIED SECTION
- Other information
- Inactive ingredients
- QUESTIONS
-
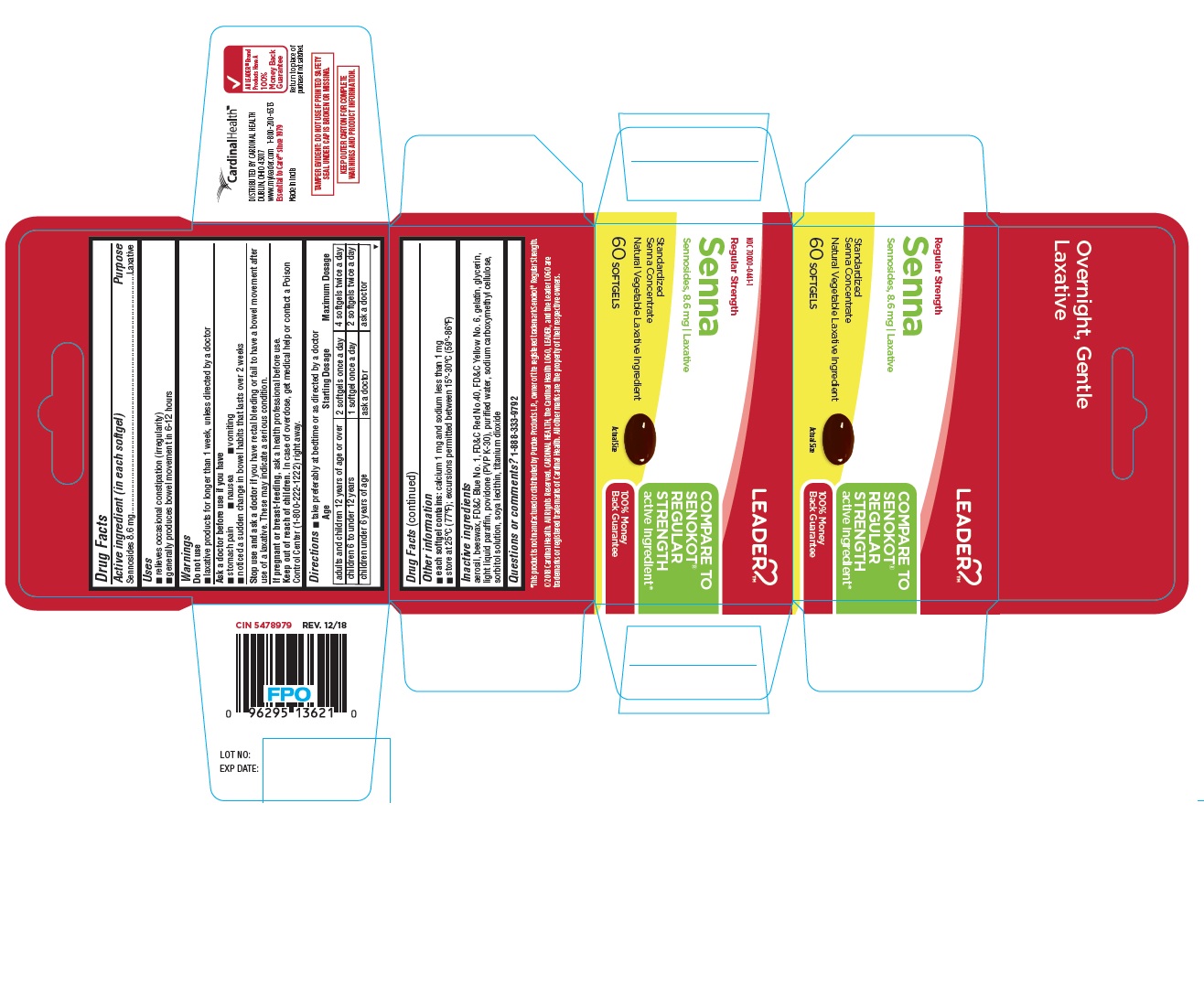
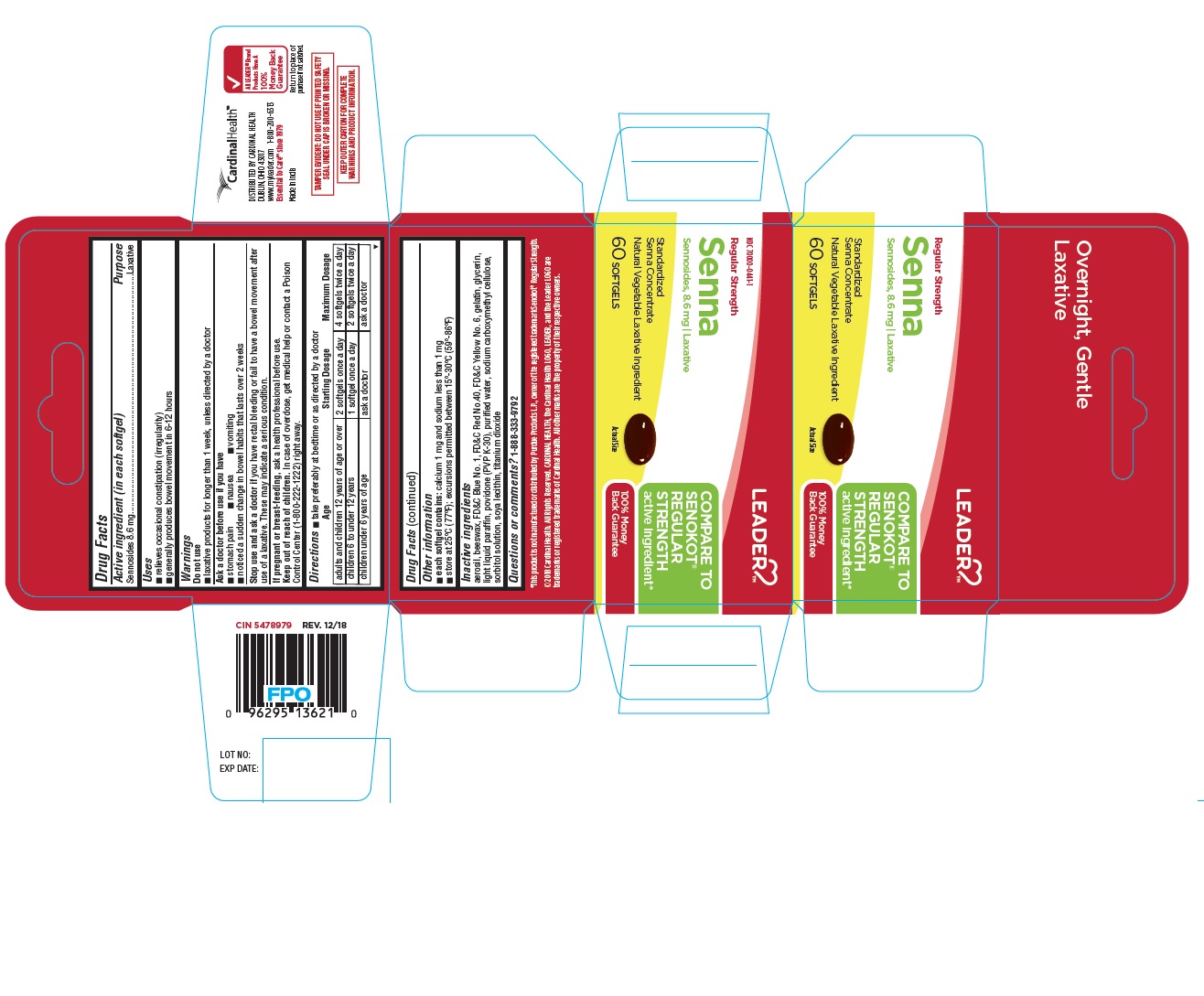
PRINCIPAL DISPLAY PANEL - LEADER™ REGULAR STRNGTH SENNA 60 Softgels Bottle Carton
LEADER
COMPARE TO
SENOKOT®
REGULAR
STRENGTH
active ingredient*
100% Money
Back GuaranteeNDC 70000-0441-1
Regular Strength
Senna
Sennosides, 8.6 mg | Laxative
Standardized
Senna Concentrate
Natural Vegetable Laxative Ingredient60 SOFTGELS
Actual Size
*This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Senokot® Regular Strength.
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0441 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) YELLOW WAX (UNII: 2ZA36H0S2V) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) PARAFFIN (UNII: I9O0E3H2ZE) POVIDONE K30 (UNII: U725QWY32X) WATER (UNII: 059QF0KO0R) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SORBITOL (UNII: 506T60A25R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown (Opaque) Score no score Shape CAPSULE (SOFTGEL) Size 12mm Flavor Imprint Code 458 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0441-1 1 in 1 CARTON 12/26/2018 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 12/26/2018 Labeler - Cardinal Health (063997360)