Label: DEMECLOCYCLINE HYDROCHLORIDE- demeclocycline tablet
- NDC Code(s): 53746-554-01, 53746-555-48
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
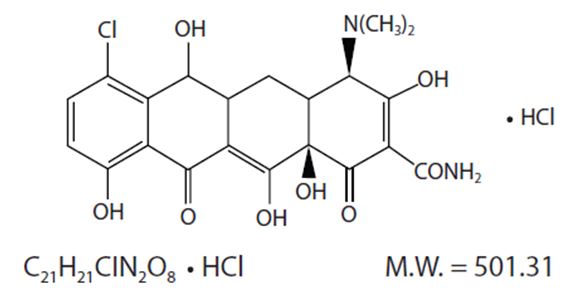
Demeclocycline hydrochloride is an antibiotic isolated from a mutant strain of Streptomyces aureofaciens. Chemically it is ...
-
CLINICAL PHARMACOLOGY
Pharmacokinetics - The absorption of demeclocycline is slower than that of tetracycline. The time to reach the peak concentration is about 4 hours. After a 150 mg oral dose of demeclocycline ...
-
INDICATIONS AND USAGE
Demeclocycline hydrochloride tablets are indicated in the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions below: Rocky Mountain spotted ...
-
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines or any of the components of the product formulation.
-
WARNINGS
DEMECLOCYCLINE HYDROCHLORIDE, LIKE OTHER TETRACYCLINE-CLASS ANTIBIOTICS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY, OR IF THE PATIENT ...
-
PRECAUTIONS
General - Pseudotumor cerebri (benign intracranial hypertension) in adults has been associated with the use of tetracyclines. The usual clinical manifestations are headache and blurred vision ...
-
ADVERSE REACTIONS
The following reactions have been reported in patients receiving tetracyclines: Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, pancreatitis and ...
-
OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Tetracyclines are not removed in significant quantities by hemodialysis or peritoneal ...
-
DOSAGE AND ADMINISTRATION
Therapy should be continued for at least 24 to 48 hours after symptoms and fever have subsided. Concomitant therapy: Absorption of tetracyclines is impaired by antacids containing aluminum ...
-
HOW SUPPLIED
Demeclocycline Hydrochloride Tablets USP, 150 mg, are supplied as red, round, convex, film-coated tablet debossed with “AN” above “54” on one side and plain on the other side. They are available ...
-
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGY
Hyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO4 and methacycline; in ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 53746-554-01 - Demeclocycline Hydrochloride Tablets, USP 150 mg (100's) Amneal Pharmaceuticals LLC
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 53746-555-48 - Demeclocycline Hydrochloride Tablets, USP 300 mg (48's) Amneal Pharmaceuticals LLC
-
INGREDIENTS AND APPEARANCEProduct Information