Label: VIBATIV- telavancin hydrochloride injection, powder, lyophilized, for solution
- NDC Code(s): 66220-315-11, 66220-315-22, 66220-315-44
- Packager: Cumberland Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VIBATIV® (telavancin) safely and effectively. See full prescribing information for VIBATIV. VIBATIV® (telavancin) for injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN HABP/VABP PATIENTS WITH PRE-EXISTING MODERATE OR SEVERE RENAL IMPAIRMENT, NEPHROTOXICITY, POTENTIAL ADVERSE DEVELOPMENTAL OUTCOMES
- Patients with pre-existing moderate/severe renal impairment (CrCl ≤ 50 mL/min) who were treated with VIBATIV for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia (HABP/VABP) had increased mortality observed versus vancomycin. Use of VIBATIV in patients with pre-existing moderate/severe renal impairment (CrCl ≤ 50 mL/min) should be considered only when the anticipated benefit to the patient outweighs the potential risk [see Warnings and Precautions (5.1, 8.4)].
- Nephrotoxicity: New onset or worsening renal impairment has occurred. Monitor renal function in all patients [see Warnings and Precautions (5.3)].
- Embryofetal Toxicity: VIBATIV may cause fetal harm. In animal reproduction studies, adverse developmental outcomes were observed in 3 animal species at clinically relevant doses. Verify pregnancy status in females of reproductive potential prior to initiating VIBATIV. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with VIBATIV and for 2 days after the final dose [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE
1.1 Complicated Skin and Skin Structure Infections - VIBATIV is indicated for the treatment of adult patients with complicated skin and skin structure infections (cSSSI) caused by susceptible ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Complicated Skin and Skin Structure Infections - The recommended dosing for VIBATIV is 10 mg/kg administered over a 60-minute period in patients ≥18 years of age by intravenous infusion once ...
-
3 DOSAGE FORMS AND STRENGTHS
VIBATIV is supplied in single-dose vials containing 750 mg telavancin as a sterile, lyophilized powder.
-
4 CONTRAINDICATIONS
4.1 Intravenous Unfractionated Heparin Sodium - Use of intravenous unfractionated heparin sodium is contraindicated with VIBATIV administration because the activated partial thromboplastin time ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Patients with HABP/VABP and Pre-existing Moderate to Severe Renal Impairment (CrCl ≤50 mL/min) In the analysis of patients (classified by the treatment received) in ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are also discussed elsewhere in the labeling: Nephrotoxicity [see Warnings and Precautions (5.3)] Infusion-related reactions [see Warnings and ...
-
7 DRUG INTERACTIONS
7.1 Drug-Laboratory Test Interactions - Effects of Telavancin on Coagulation Test Parameters - Telavancin binds to the artificial phospholipid surfaces added to common anticoagulation tests ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on findings in animal reproduction studies, VIBATIV may cause fetal harm. There are no available data on VIBATIV use in pregnant women to evaluate for a ...
-
10 OVERDOSAGE
In the event of overdosage, VIBATIV should be discontinued and supportive care is advised with maintenance of glomerular filtration and careful monitoring of renal function. Following ...
-
11 DESCRIPTION
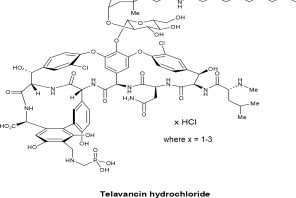
VIBATIV contains telavancin hydrochloride (Figure 1), a lipoglycopeptide antibacterial that is a synthetic derivative of vancomycin. The chemical name of telavancin hydrochloride is vancomycin ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Telavancin is an antibacterial drug [see Clinical Pharmacology (12.4)]. 12.2 Pharmacodynamics - The antimicrobial activity of telavancin appears to best correlate ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to determine the carcinogenic potential of telavancin have not been performed. Neither mutagenic nor ...
-
14 CLINICAL STUDIES
14.1 Complicated Skin and Skin Structure Infections - Adult patients with clinically documented complicated skin and skin structure infections (cSSSI) were enrolled in two randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
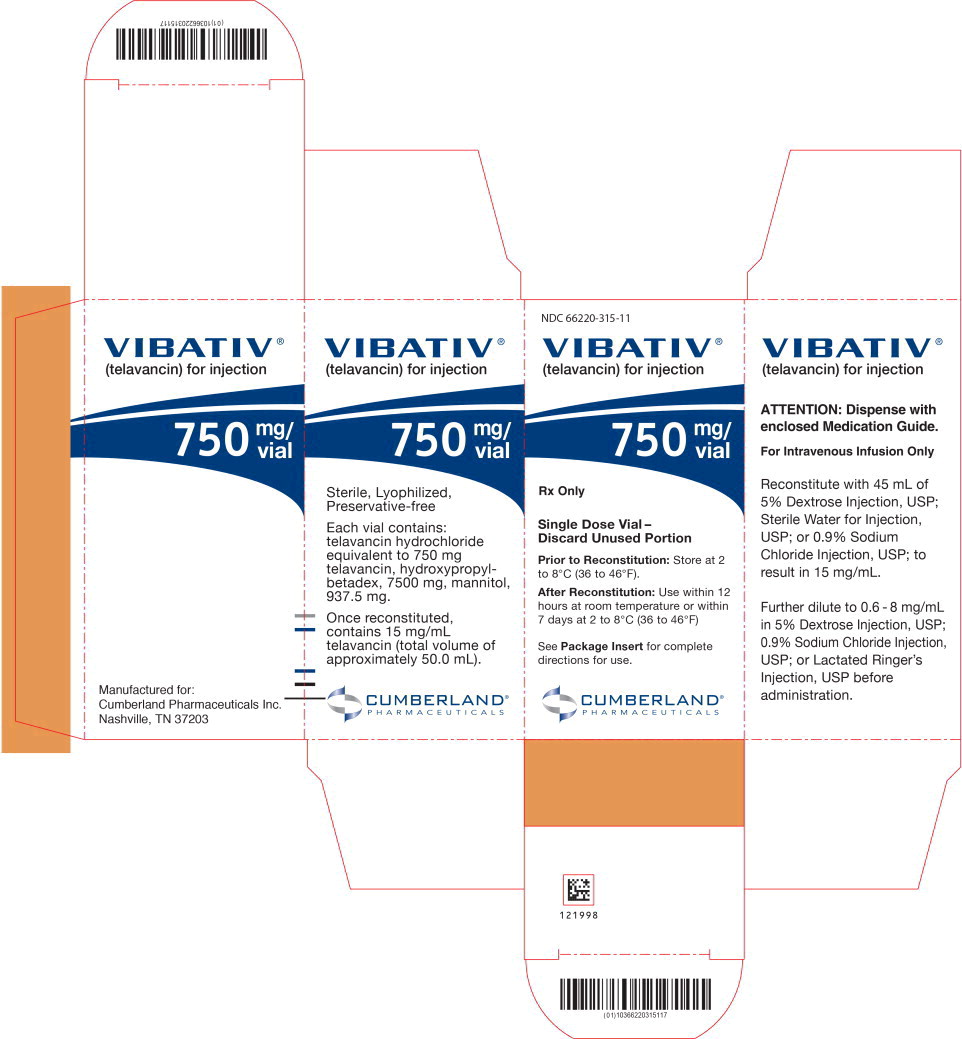
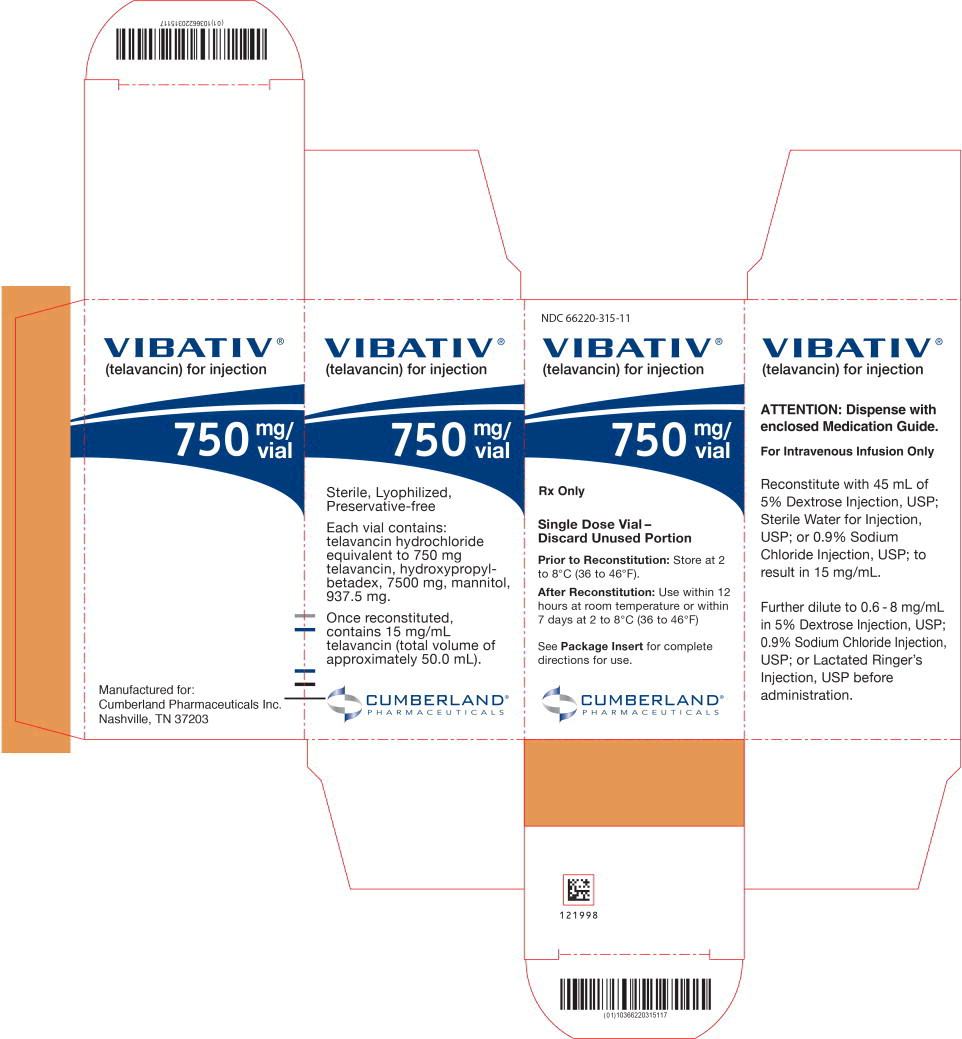
Individual cartons of 750 mg single-dose vials (NDC 66220-315-11) Cartons of 12 individually packaged 750 mg single-dose vials (NDC 66220-315-22); cartons of 4 individually packaged 750 mg ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Embryo-Fetal Toxicity - Advise pregnant women and females of reproductive potential of the potential risk to a ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration - Revised: 01/2021 - MEDICATION GUIDE - VIBATIV® (vy-'ba-tiv) (telavancin) for injection, for ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 750 mg/vial Carton Label - NDC 66220-315-11 - VIBATIV® (telavancin) for injection - 750 mg/ vial - Rx Only - Single Dose Vial- Discard Unused Portion - Prior to ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 750 mg/vial Vial Label - NDC 66220-315-11 - VIBATIV® (telavancin) for injection - 750 mg/vial - For Intravenous Infusion Only - Single Dose Vial- Discard Unused ...
-
INGREDIENTS AND APPEARANCEProduct Information