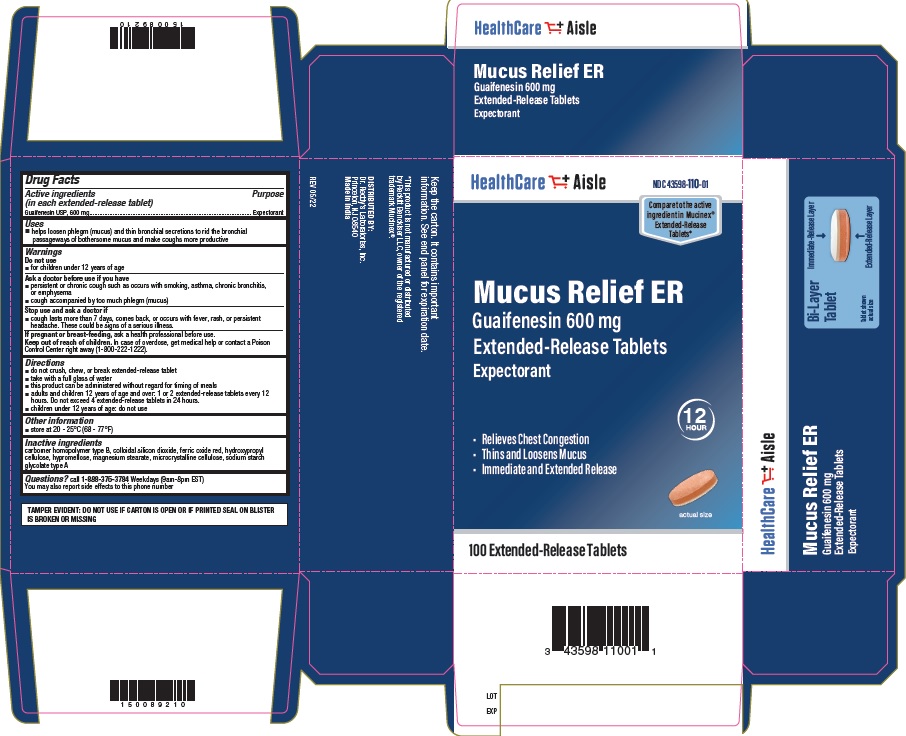
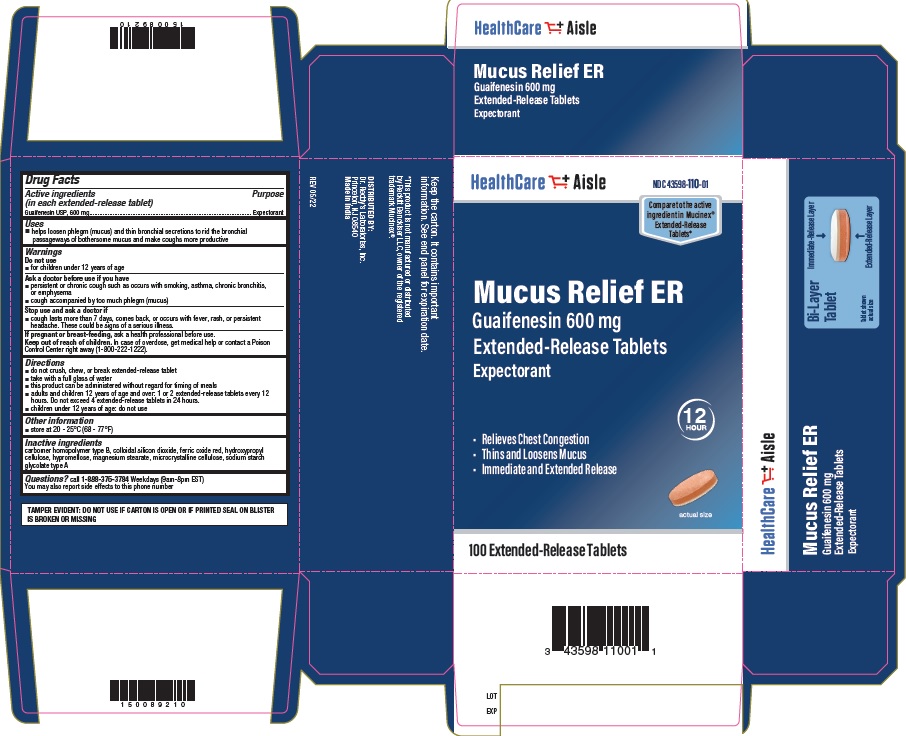
Label: GUAIFENESIN 600 MG- guaifenesin tablet, extended release
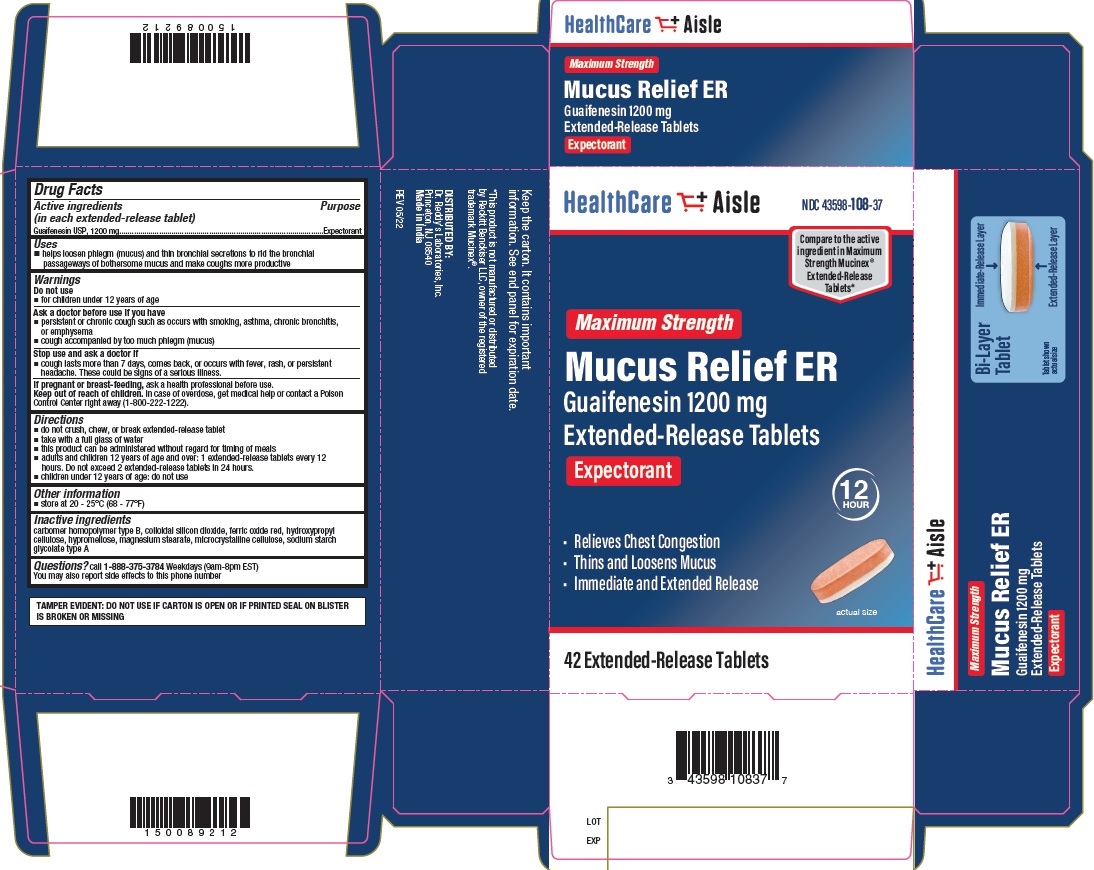
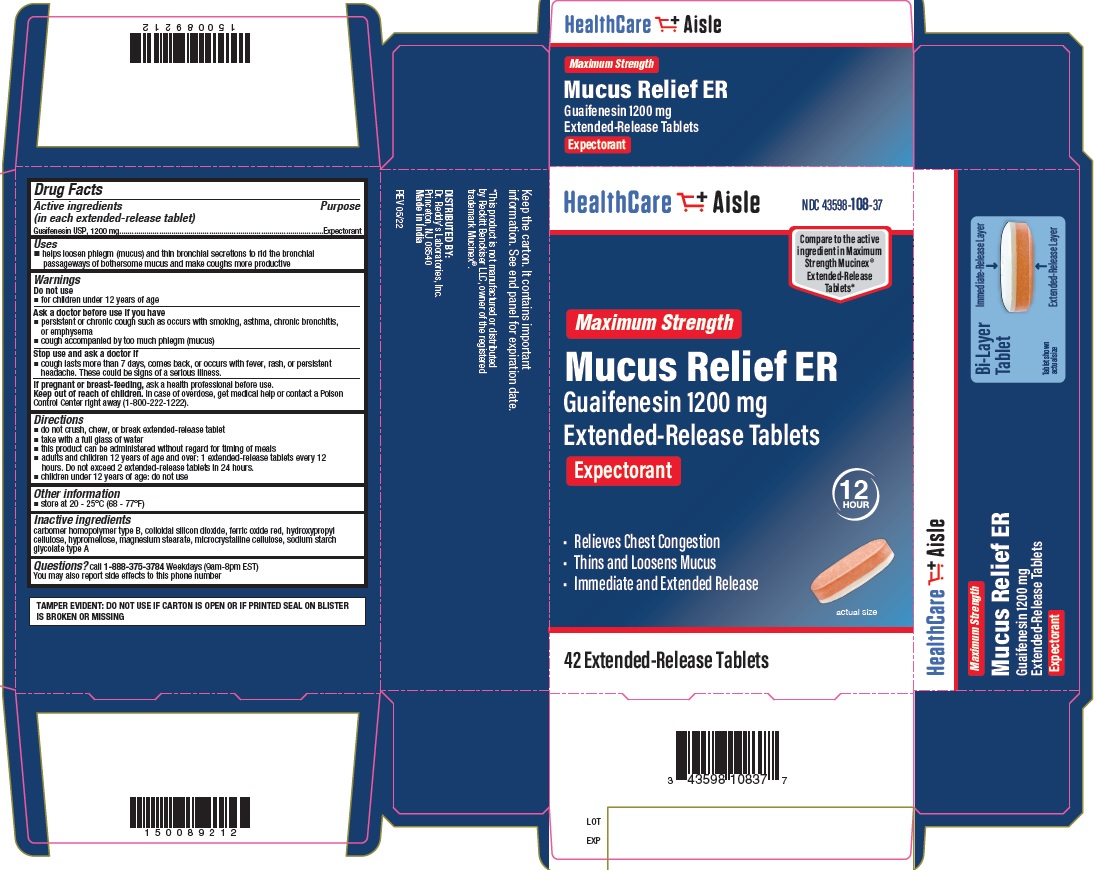
GUAIFENESIN 1200 MG- guaifenesin tablet, extended release
- NDC Code(s): 43598-108-37, 43598-110-01
- Packager: Dr. Reddy's Laboratories Inc.
- This is a repackaged label.
- Source NDC Code(s): 43598-008, 43598-009
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
- Use(s)
-
Warnings
Applicable warning(s) in 201.66(c)(5)(i) and (ii)
Ask a doctor before use if
-
persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
-
cough accompanied by too much phlegm (mucus)
-
-
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1or 2 extended-release tablets every 12 hours. Do not exceed 4 extended-release tablets in 24 hours (for 600 mg)
- adults and children 12 years of age and over: 1 extended-release tablet every 12 hours. Do not exceed 2 extended-release tablets in 24 hours (for 1200 mg)
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Principal Display Panel- 600mg Carton
- Principal Display Panel - 1200 mg Carton
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN 600 MG
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43598-110(NDC:43598-008) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 600 mg Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) Silicon Dioxide (UNII: ETJ7Z6XBU4) Ferric Oxide Red (UNII: 1K09F3G675) Hydroxypropyl Cellulose (110000 Wamw) (UNII: 5Y0974F5PW) Hypromellose 2910 (10000 Mpa.S) (UNII: 0HO1H52958) Hypromellose 2208 (4000 Mpa.S) (UNII: 39J80LT57T) Magnesium Stearate (UNII: 70097M6I30) Microcrystalline Cellulose (UNII: OP1R32D61U) Sodium Starch Glycolate Type A Potato (UNII: 5856J3G2A2) Product Characteristics Color PINK (White on debossed side and Light Pink to Pink on other side ) Score no score Shape OVAL Size 16mm Flavor Imprint Code 6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43598-110-01 5 in 1 CARTON 09/01/2022 1 20 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215932 09/01/2022 GUAIFENESIN 1200 MG
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43598-108(NDC:43598-009) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 1200 mg Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) Silicon Dioxide (UNII: ETJ7Z6XBU4) Ferric Oxide Red (UNII: 1K09F3G675) Hydroxypropyl Cellulose (110000 Wamw) (UNII: 5Y0974F5PW) Hypromellose 2910 (10000 Mpa.S) (UNII: 0HO1H52958) Hypromellose 2208 (4000 Mpa.S) (UNII: 39J80LT57T) Magnesium Stearate (UNII: 70097M6I30) Microcrystalline Cellulose (UNII: OP1R32D61U) Sodium Starch Glycolate Type A Potato (UNII: 5856J3G2A2) Product Characteristics Color PINK (White on debossed side and Light Pink to Pink on other side ) Score no score Shape OVAL Size 22mm Flavor Imprint Code 12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43598-108-37 3 in 1 CARTON 09/01/2022 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215932 09/01/2022 Labeler - Dr. Reddy's Laboratories Inc. (802315887)