Label: SUCRALFATE tablet
- NDC Code(s): 82804-102-30
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 29033-003
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
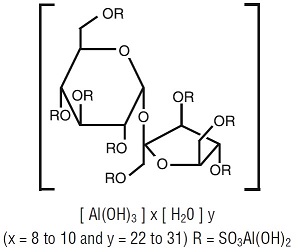
DESCRIPTIONSucralfate is an α-D-glucopyranoside, β-D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula: Tablets for oral administration contain 1 g of ...
-
CLINICAL PHARMACOLOGYSucralfate is only minimally absorbed from the gastrointestinal tract. The small amounts of the sulfated disaccharide that are absorbed are excreted primarily in the urine. Although the mechanism ...
-
Clinical TrialsAcute Duodenal Ulcer - Over 600 patients have participated in well-controlled clinical trials worldwide. Multicenter trials conducted in the United States, both of them placebo-controlled studies ...
-
INDICATIONS AND USAGESucralfate tablets are indicated in: • Short-term treatment (up to 8 weeks) of active duodenal ulcer. While healing with sucralfate may occur during the first week or two, treatment should be ...
-
CONTRAINDICATIONSSucralfate tablets are contraindicated in patients with known hypersensitivity reactions to the active substance or to any of the excipients.
-
PRECAUTIONSThe physician should read the "PRECAUTIONS" section when considering the use of this drug in pregnant or pediatric patients, or patients of childbearing potential. Duodenal ulcer is a chronic ...
-
ADVERSE REACTIONSAdverse reactions to sucralfate in clinical trials were minor and only rarely led to discontinuation of the drug. In studies involving over 2700 patients treated with sucralfate tablets, adverse ...
-
OVERDOSAGEDue to limited experience in humans with overdosage of sucralfate, no specific treatment recommendations can be given. Acute oral toxicity studies in animals, however, using doses up to 12 g/kg ...
-
DOSAGE AND ADMINISTRATIONActive Duodenal Ulcer. The recommended adult oral dosage for duodenal ulcer is 1 g four times per day on an empty stomach. Antacids may be prescribed as needed for relief of pain but should not ...
-
HOW SUPPLIEDSucralfate 1 g tablets, USP are supplied in bottles of 30. White, oblong, bisected tablets debossed with "N'' and ''S1'' on one side. Bottles of 30...........NDC 82804-102-30 - Store at 20° to 25°C ...
-
PRINCIPAL DISPLAY PANELNDC 82804-102-30 - Sucralfate - Tablets, USP - 1 gram - Rx Only - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information