Label: LABETALOL HYDROCHLORIDE tablet, film coated
- NDC Code(s): 70518-3993-0, 70518-3993-1, 70518-3993-2
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 70377-061
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
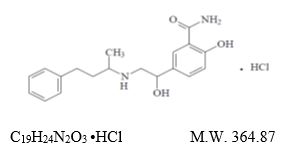
DESCRIPTIONLabetalol Hydrochloride Tablets, USP are adrenergic receptor blocking agents that have both selective alpha - 1-adrenergic and non-selective - beta-adrenergic receptor blocking actions in a ...
-
CLINICAL PHARMACOLOGYLabetalol HCl combines both selective, competitive alpha - 1-adrenergic blocking and nonselective, competitive beta-adrenergic blocking activity in a single substance. In man, the ratios of ...
-
INDICATIONS AND USAGELabetalol HCl tablets are indicated in the management of hypertension. Labetalol HCl tablets may be used alone or in combination with other antihypertensive agents, especially thiazide and loop ...
-
CONTRAINDICATIONSLabetalol HCl tablets are contraindicated in bronchial asthma, overt cardiac failure, greater-than-first degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with ...
-
WARNINGSHepatic Injury - Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis ...
-
PRECAUTIONSGeneral - Impaired Hepatic Function - Labetalol HCl tablets should be used with caution in patients with impaired hepatic function since metabolism of the drug may be diminished. Intraoperative ...
-
ADVERSE REACTIONSMost adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months' duration, discontinuation of labetalol HCl tablets due to ...
-
OVERDOSAGEOverdosage with labetalol HCl causes excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary to ...
-
DOSAGE AND ADMINISTRATIONDOSAGE MUST BE INDIVIDUALIZED. The recommended - initialdosage is 100 mg - twicedaily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an ...
-
HOW SUPPLIEDLabetalol Hydrochloride Tablets, USP 200 mg are available as white to off-white, round biconvex tablets, debossed “AC 371” on one side and bisected on other side, packaged in - NDC ...
-
PRINCIPAL DISPLAY PANELDRUG: Labetalol Hydrochloride - GENERIC: Labetalol Hydrochloride - DOSAGE: TABLET, FILM COATED - ADMINSTRATION: ORAL - NDC: 70518-3993-0 - NDC: 70518-3993-1 - NDC: 70518-3993-2 - COLOR: white - SHAPE: ROUND - SCORE ...
-
INGREDIENTS AND APPEARANCEProduct Information