Label: DEXTROSE- dextrose monohydrate injection
- NDC Code(s): 0338-0204-04, 0338-0208-04, 0338-0211-04, 0338-0216-04
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DEXTROSE INJECTION safely and effectively. See full prescribing information for DEXTROSE INJECTION. DEXTROSE Injection, for ...
-
Table of ContentsTable of Contents
-
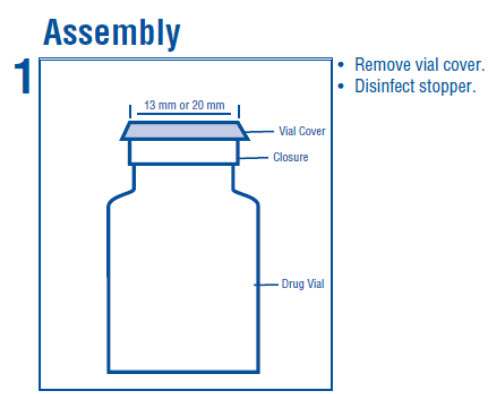
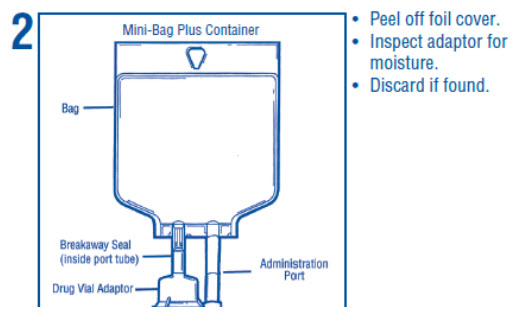
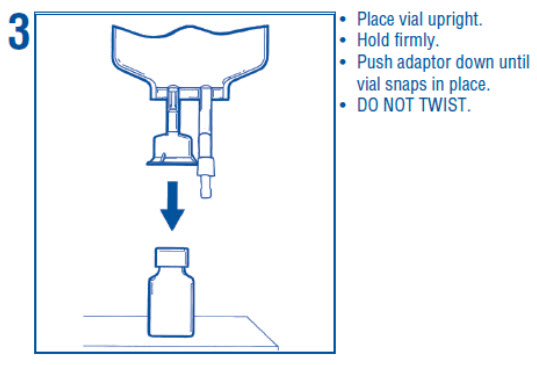
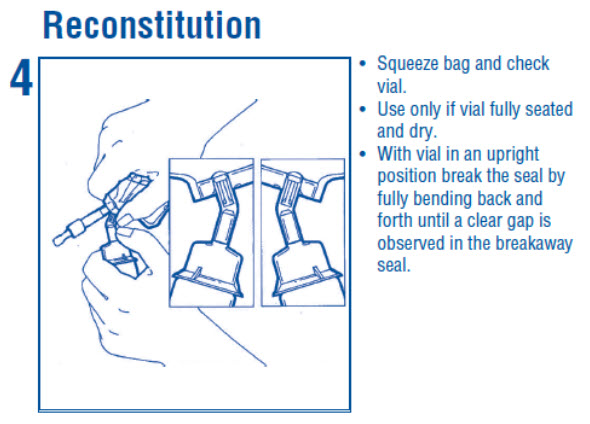
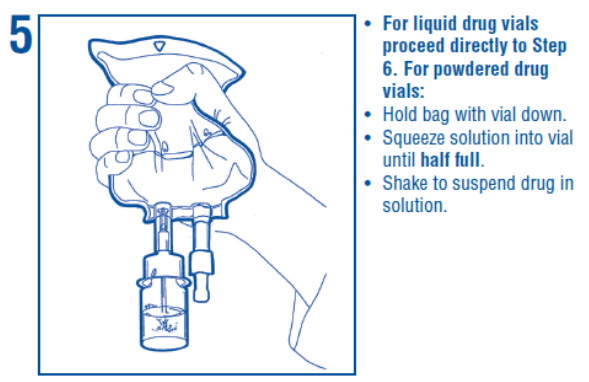
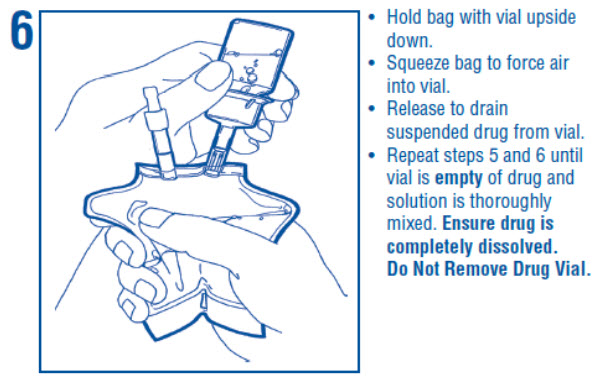
1 INDICATIONS AND USAGE Dextrose Injection is indicated as source of water and calories and may also be used as diluent for reconstitution of a powdered or liquid (up to 10 mL) drug product packaged in a vial with a 13 ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions - • Dextrose Injection is intended for intravenous use. • Peripheral administration of 5% dextrose is generally acceptable, however, consider central ...
-
3 DOSAGE FORMS AND STRENGTHS Dextrose Injection, USP is a clear, sterile, non-pyrogenic solution supplied as 5 grams of dextrose hydrous per 100 mL (0.05 grams/mL) in 50 mL and 100 mL single-dose, flexible containers.
-
4 CONTRAINDICATIONS The use of Dextrose Injection is contraindicated in patients with: • Clinically significant hyperglycemia [see Warnings and Precautions (5.1)]. • Known hypersensitivity to dextrose [see Warnings ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hyperglycemia and Hyperosmolar Hyperglycemic State - The use of dextrose infusions in patients with impaired glucose tolerance may worsen hyperglycemia. Administration of dextrose at a rate ...
-
6 ADVERSE REACTIONS The following adverse reactions associated with the use of Dextrose Injection were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a ...
-
7 DRUG INTERACTIONS 7.1 Other Products that Affect Glycemic Control or Fluid and/or Electrolyte Balance - Dextrose Injection can affect glycemic control, vasopressin and fluid and/or electrolyte balance [see ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Appropriate administration of Dextrose Injection during pregnancy is not expected to cause adverse developmental outcomes, including congenital malformations ...
-
10 OVERDOSAGE An increased infusion rate of Dextrose Injection or administration of dextrose solutions can cause hyperglycemia, hyperosmolality, and adverse effects on water and electrolyte balance [see ...
-
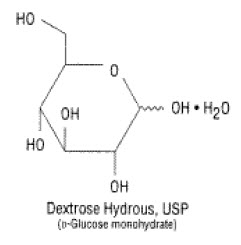
11 DESCRIPTION Dextrose Injection, 5% USP is a clear, sterile, non-pyrogenic solution of Dextrose, USP in Water for Injection in a polyvinylchloride flexible plastic container for intravenous administration ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Dextrose oxidized to carbon dioxide and water, yielding energy.
-
16 HOW SUPPLIED/STORAGE AND HANDLING Dextrose Injection 5%, USP is a clear, sterile solution of dextrose supplied in single-dose, flexible containers. The product is available as quad packs (4 units per overpouch). Table 2 lists quad ...
-
17 PATIENT COUNSELING INFORMATION Inform patients, caregivers, or home healthcare providers of the following risks of Dextrose Injection: Hyperglycemia and hyperosmolar hyperglycemic state - Inform patients and their caregivers ...
-
SPL UNCLASSIFIED SECTIONManufactured by, Packed by, Distributed by: Baxter Healthcare Corporation - Deerfield, IL 60015 USA - Printed in USA - 07-19-00-4103 - Baxter, Mini-Bag Plus, and Viaflex are trademarks of Baxter ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL LOT EXP - 5% Dextrose - Injection USP - MINI-BAG Plus Container - FOR USE WITH 13 MM VIAL - 2B0036LF - NDC 0338-0204-04 - 1 Symbol - 13 - mm - VIAL - Each 50 mL contains 2.5 g Dextrose Hydrous - USP pH 4.0 ...
-
INGREDIENTS AND APPEARANCEProduct Information