Label: AUCATZYL- obecabtagene autoleucel kit
- NDC Code(s): 83047-010-10, 83047-100-10, 83047-100-30, 83047-300-30, view more
- Packager: Autolus Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AUCATZYL safely and effectively. See full prescribing information for AUCATZYL. AUCATZYL® (obecabtagene autoleucel) suspension ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), occurred in patients receiving AUCATZYL. Do not administer AUCATZYL to patients with active infection or inflammatory disorders. Prior to administering AUCATZYL, ensure that healthcare providers have immediate access to medications and resuscitative equipment to manage CRS [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), including fatal or life-threatening reactions, occurred in patients receiving AUCATZYL, including concurrently with CRS or after CRS resolution. Monitor for neurologic signs and symptoms after treatment with AUCATZYL. Prior to administering AUCATZYL, ensure that healthcare providers have immediate access to medications and resuscitative equipment to manage neurologic toxicities. Provide supportive care and/or corticosteroids, as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies [see Warnings and Precautions (5.8)].
-
1 INDICATION AND USAGEAUCATZYL is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia ...
-
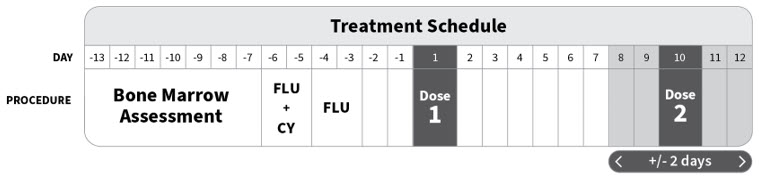
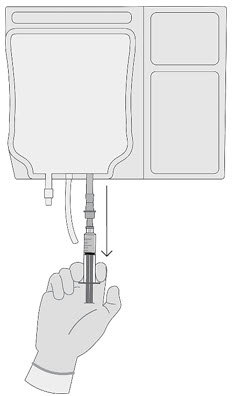
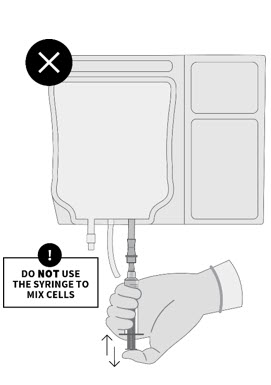
2 DOSAGE AND ADMINISTRATIONFor autologous use only. For intravenous use only. Strictly follow Administration instructions to minimize dosing errors [see Overdosage (10)]. 2.1 Dose - The total recommended dose of ...
-
3 DOSAGE FORMS AND STRENGTHSAUCATZYL is a cell suspension for infusion. AUCATZYL contains a total recommended dose of 410 × 106 CD19 CAR-positive viable T cells supplied in three to five infusion bags. The infusion volume is ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Cytokine Release Syndrome - Cytokine Release Syndrome (CRS) occurred following treatment with AUCATZYL. CRS was reported in 75% (75/100) of patients including Grade 3 CRS in 3% of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There is limited available data with AUCATZYL use in pregnant women. In the FELIX study, one patient became pregnant 6 months following treatment with AUCATZYL ...
-
10 OVERDOSAGEIn FELIX Study (all cohorts N=127), occurrences of overdose were observed at the administration of the first dose in 4% (5/127) of patients. All 5 patients had bone marrow blasts > 20% and should ...
-
11 DESCRIPTIONAUCATZYL (obecabtagene autoleucel) is a CD19-directed genetically modified autologous T cell immunotherapy comprised of the patient's T cells that are transduced with a lentiviral vector to ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - AUCATZYL is a CD19-directed genetically modified autologous T cell immunotherapy consisting of the patient's own T cells expressing an anti-CD19 CAR. Engagement of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No in vivo or in vitro carcinogenicity, mutagenicity or genotoxicity studies have been conducted with AUCATZYL. No studies have been ...
-
14 CLINICAL STUDIESThe efficacy of AUCATZYL was evaluated in an open-label, multi-center, single-arm study (FELIX study; NCT04404660). The study enrolled patients with relapsed or refractory B-cell acute ...
-
15 REFERENCESLee DW et al (2019), ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019 Apr ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAUCATZYL 410 × 106 CD19 CAR-positive viable T cells NDC (83047-410-04) is supplied in three to five infusion bags (see below) containing a frozen suspension of genetically modified autologous T ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Discuss the following with the patient: Inform patients that there is a risk of manufacturing failure (4.5% [5/112 ...
-
MEDICATION GUIDEMEDICATION GUIDE - AUCATZYL® [pronounced aw-kat-zil] (obecabtagene autoleucel) Read this Medication Guide before you start your AUCATZYL treatment. The more you know about your treatment, the ...
-
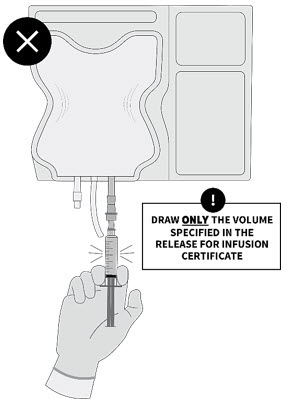
PRINCIPAL DISPLAY PANEL - 10 mL Infusion Bag Label - 10 x 1010x106 bag configuration - EXTRACT SPECIFIED VOLUME VIA SYRINGE - obecabtagene autoleucel - AUCATZYL® FOR AUTOLOGOUS & INTRAVENOUS USE ONLY. Dosage: See prescribing information and release for - infusion ...
-
PRINCIPAL DISPLAY PANEL - 10 mL to 20 mL Infusion Bag Label - 100 x 10100x106 bag configuration - obecabtagene autoleucel - AUCATZYL® FOR AUTOLOGOUS & INTRAVENOUS USE ONLY. Dosage: See prescribing information and the release for - infusion certificate. Contains: 100x106 ...
-
PRINCIPAL DISPLAY PANEL - 30 mL to 70 mL Infusion Bag Label - 100 x 10100x106 bag configuration - obecabtagene autoleucel - AUCATZYL® FOR AUTOLOGOUS & INTRAVENOUS USE ONLY. Dosage: See prescribing information and the release for - infusion certificate. Contains: 100x106 ...
-
PRINCIPAL DISPLAY PANEL - 30 mL to 70 mL Infusion Bag Label - 300 x 10300x106 bag configuration - obecabtagene autoleucel - AUCATZYL® FOR AUTOLOGOUS & INTRAVENOUS USE ONLY. Dosage: See prescribing information and the release for - infusion certificate. Contains: 300x106 ...
-
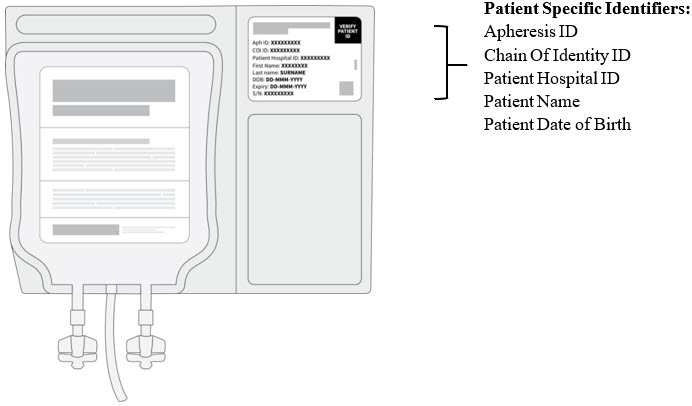
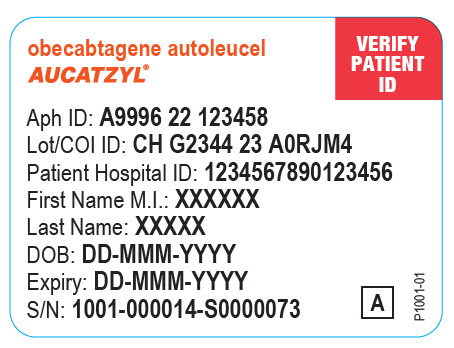
PRINCIPAL DISPLAY PANEL - Patient Information Labelobecabtagene autoleucel - AUCATZYL® VERIFY - PATIENT - ID - Aph ID: Lot/COI ID: Patient Hospital ID: First Name M.I.: Last Name: DOB: DD-MMM-YYYY - Expiry: DD-MMM-YYYY - S/N: A - P1001-01
-
PRINCIPAL DISPLAY PANEL - Kit Labelobecabtagene autoleucel - AUCATZYL® FOR AUTOLOGOUS & INTRAVENOUS USE ONLY. Dose: 410 x 106 CD19 CAR-positive viable T cells - Dosage: See prescribing information and the release for infusion ...
-
INGREDIENTS AND APPEARANCEProduct Information