Label: ETOPOSIDE injection
- NDC Code(s): 16729-114-08, 16729-114-11, 16729-114-31, 16729-114-32
- Packager: Accord Healthcare Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx ONLY - WARNINGS - Etoposide should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe myelosuppression with ...
-
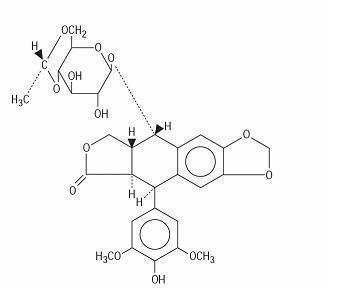
DESCRIPTIONEtoposide (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin ...
-
CLINICAL PHARMACOLOGYEtoposide Injection USP has been shown to cause metaphase arrest in chick fibroblasts. Its main effect, however, appears to be at the G2 portion of the cell cycle in mammalian cells. Two different ...
-
INDICATIONS AND USAGEEtoposide Injection USP is indicated in the management of the following neoplasms: Refractory Testicular Tumors - Etoposide Injection USP in combination therapy with other approved ...
-
CONTRAINDICATIONSEtoposide Injection USP is contraindicated in patients who have demonstrated a previous hypersensitivity to etoposide or any component of the formulation.
-
WARNINGSPatients being treated with etoposide Injection USP must be frequently observed for myelosuppression both during and after therapy. Myelosuppression resulting in death has been reported ...
-
PRECAUTIONSGeneral - In all instances where the use of etoposide Injection USP is considered for chemotherapy, the physician must evaluate the need and usefulness of the drug against the risk of adverse ...
-
ADVERSE REACTIONSThe following data on adverse reactions are based on intravenous administration of etoposide Injection USP as a single agent, using several different dose schedules for treatment of a wide variety ...
-
OVERDOSAGENo proven antidotes have been established for etoposide Injection USP overdosage.
-
DOSAGE AND ADMINISTRATIONNote: Plastic devices made of acrylic or ABS (a polymer composed of acrylonitrile, butadiene, and styrene) have been reported to crack and leak when used with - undilutedEtoposide Injection ...
-
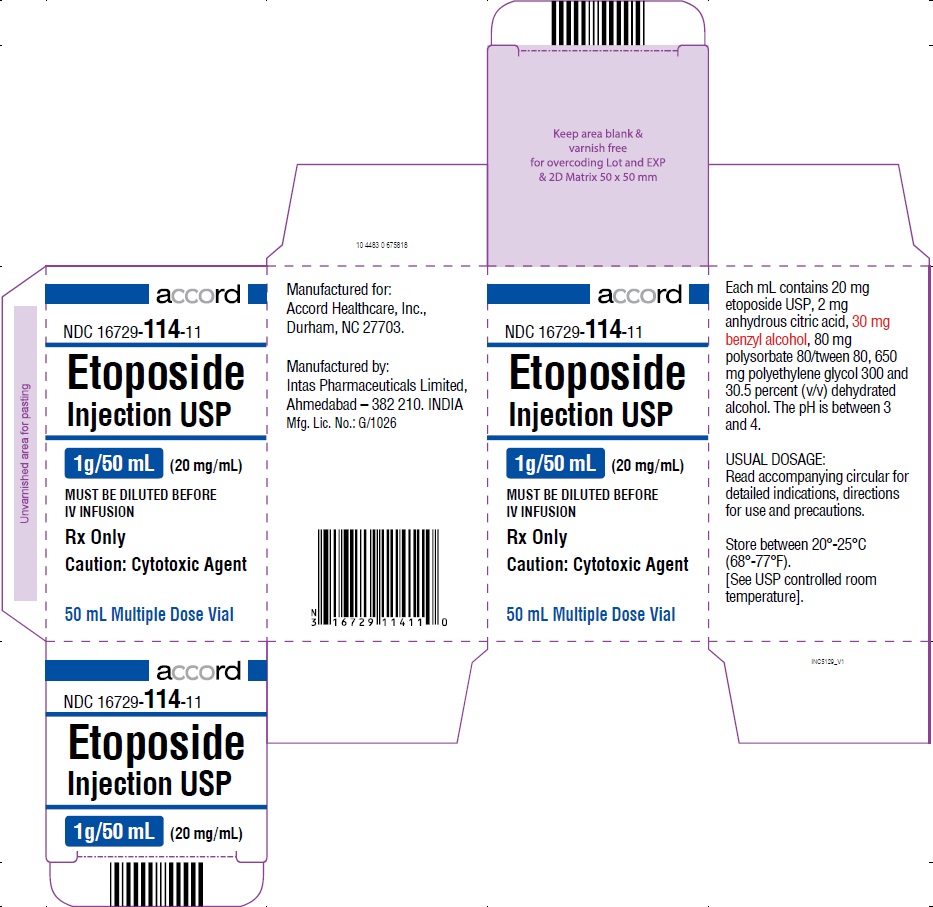
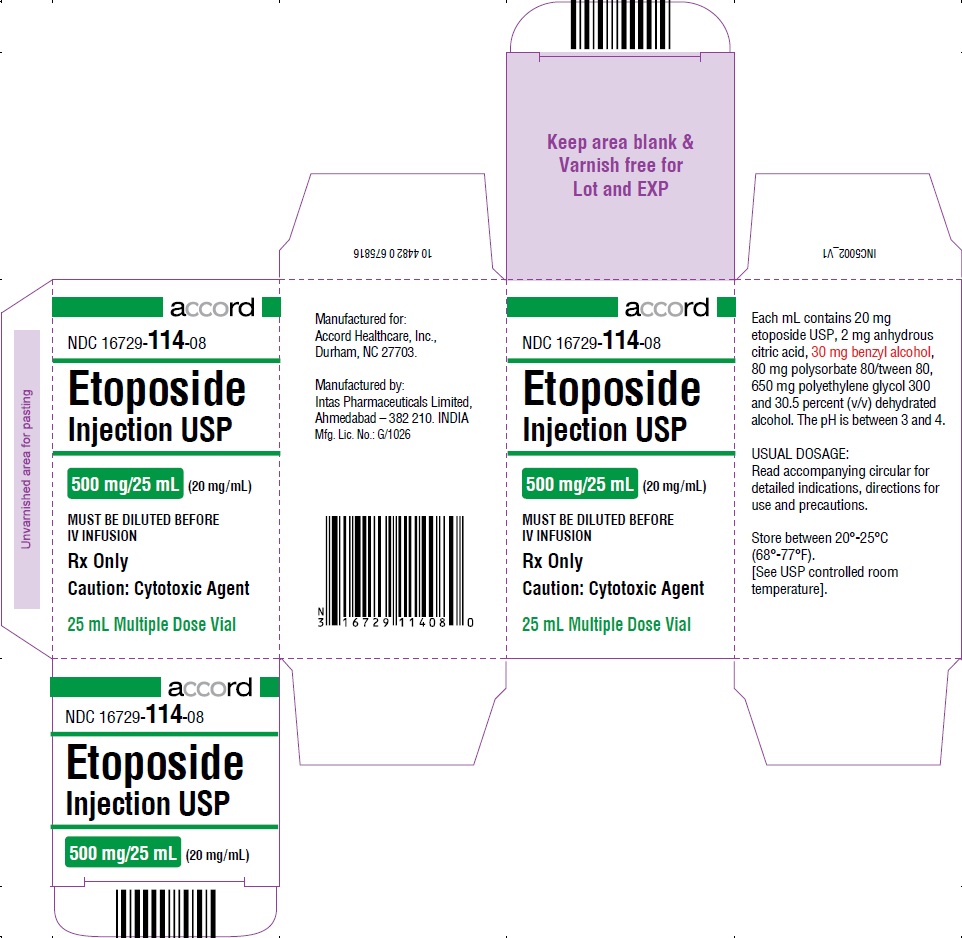
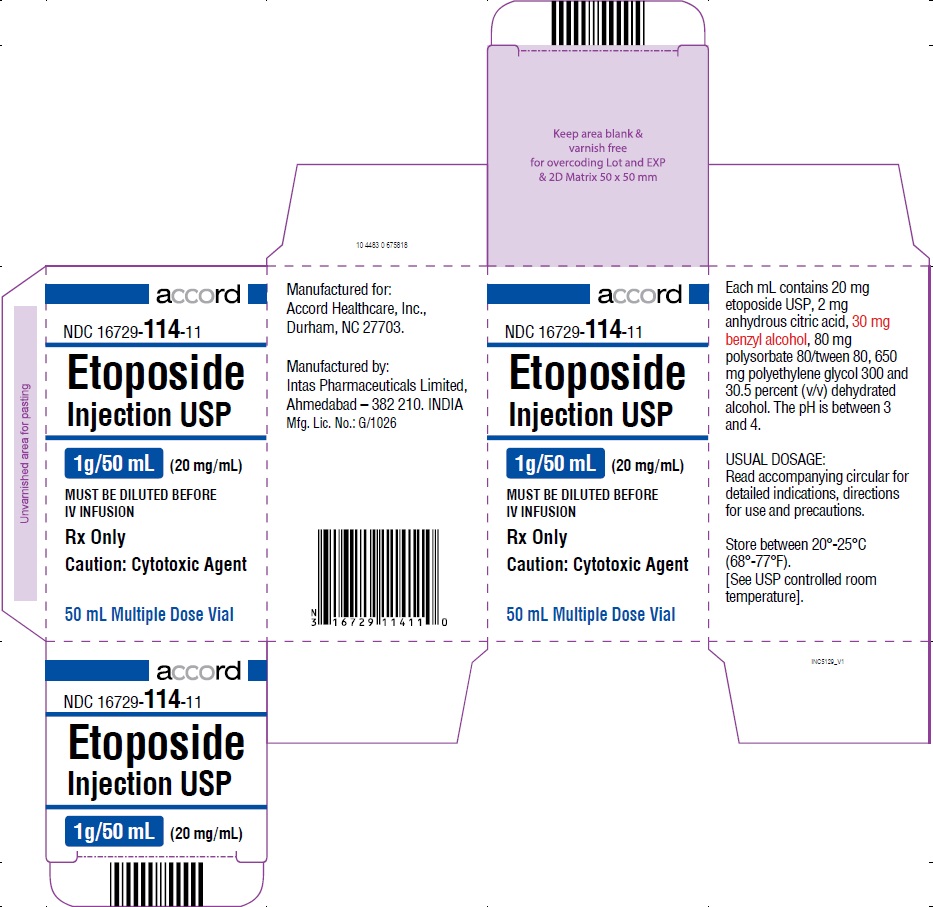
HOW SUPPLIEDEtoposide Injection USP, 20 mg/mL, is supplied as follows: NDC 16729-114-31 100 mg/5 mL Sterile, Multiple Dose Vial. NDC 16729-114-32 250 mg/12.5 mL Sterile ...
-
REFERENCESRecommendations for the Safe Handling of Parenteral Antineoplastic Drugs. NIH Publication No. 83-2621. For sale by the Superintendent of Documents, US Government Printing Office, Washington, DC ...
-
PRINCIPAL DISPLAY PANEL-100 mg/5 mL Carton Label

-
PRINCIPAL DISPLAY PANEL-500 mg/12.5 mL Carton Label

-
PRINCIPAL DISPLAY PANEL-500 mg/25 mL Carton Label
-
PRINCIPAL DISPLAY PANEL-1 g/50 mL Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information