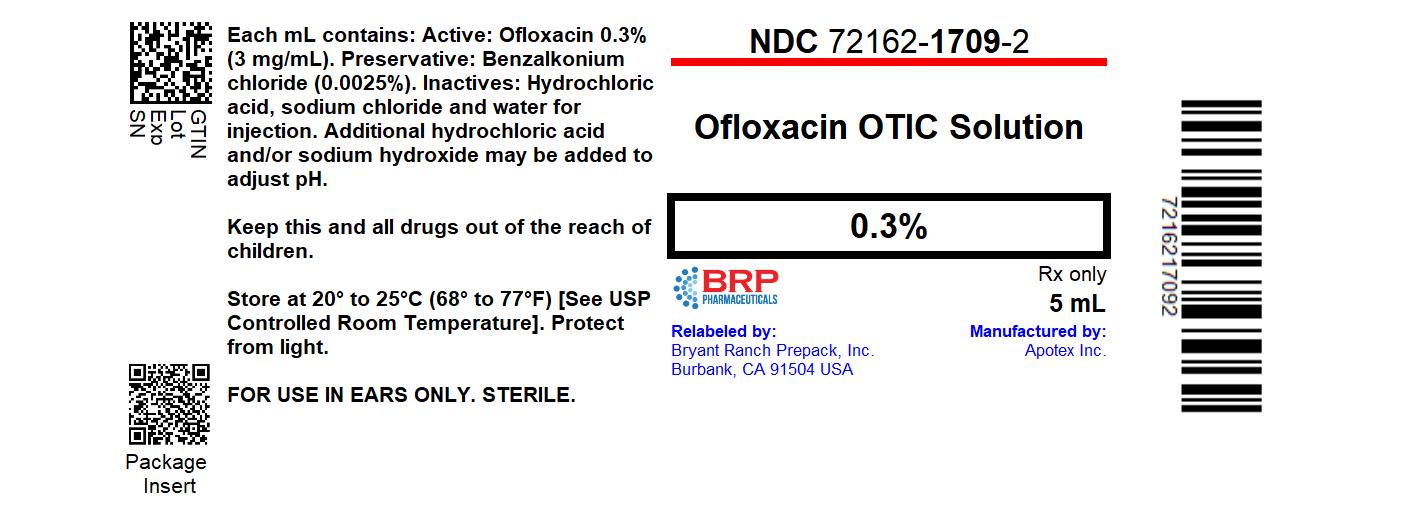
Label: OFLOXACIN OTIC- ofloxacin solution
- NDC Code(s): 72162-1709-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 60505-0363
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
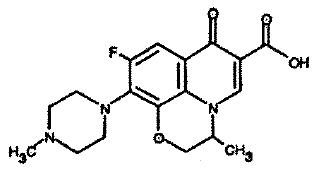
DESCRIPTIONOfloxacin Otic Solution 0.3% is a sterile aqueous anti-infective (anti-bacterial) solution for otic use. Chemically, ofloxacin has three condensed 6-membered rings made up of a fluorinated ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - Drug concentrations in serum (in subjects with tympanostomy tubes and perforated tympanic membranes), in otorrhea, and in mucosa of the middle ear (in subjects with perforated ...
-
INDICATIONS AND USAGEOfloxacin otic solution 0.3% is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below: Otitis Externa ...
-
CONTRAINDICATIONSOfloxacin otic solution 0.3% is contraindicated in patients with a history of hypersensitivity to ofloxacin, to other quinolones, or to any of the components in this medication.
-
WARNINGSNOT FOR OPHTHALMIC USE. NOT FOR INJECTION. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving ...
-
PRECAUTIONSGeneral - As with other anti-infective preparations, prolonged use may result in over-growth of nonsusceptible organisms, including fungi. If the infection is not improved after one week ...
-
ADVERSE REACTIONSSubjects with Otitis Externa - In the phase III clinical trials performed in support of once-daily dosing, 799 subjects with otitis externa and intact tympanic membranes were treated with ...
-
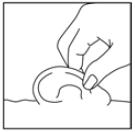
DOSAGE AND ADMINISTRATIONOtitis Externa - The recommended dosage regimen for the treatment of otitis externa is: For pediatric patients (from 6 months to 13 years old): Five drops (0.25 mL, 0.75 mg ofloxacin) instilled ...
-
HOW SUPPLIEDOfloxacin Otic Solution 0.3% is supplied in a bottle containing 5 mL. NDC: 72162-1709-2: 5 mL in a BOTTLE, DROPPER - Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature] ...
-
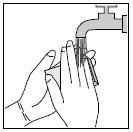
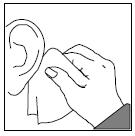
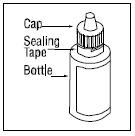
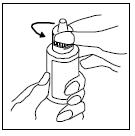
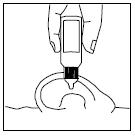
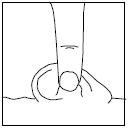
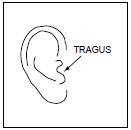
PATIENT INFORMATIONRx Only - Ofloxacin Otic Solution, 0.3% IMPORTANT PATIENT INFORMATION AND INSTRUCTIONS. READ BEFORE USE. What is Ofloxacin Otic Solution? Ofloxacin Otic Solution is an antibiotic in a ...
-
PRINCIPAL DISPLAY PANELOfloxacin OTIC Solution #5
-
INGREDIENTS AND APPEARANCEProduct Information