Label: TESTOSTERONE gel
-
NDC Code(s):
0591-2924-18,
0591-2925-30,
0591-2925-32,
0591-2926-25, view more0591-2926-30
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 29, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TESTOSTERONE GEL safely and effectively. See full prescribing information for TESTOSTERONE GEL.
TESTOSTERONE gel 1.62% for topical use CIII
Initial U.S. Approval: 1953
WARNING: SECONDARY EXPOSURE TO TESTOSTERONE
See full prescribing information for complete boxed warning.
-
Virilization has been reported in children who were secondarily exposed to testosterone gel. (5.2, 6.2)
-
Children should avoid contact with unwashed or unclothed application sites in men using testosterone gel. (2.2, 5.2)
- Healthcare providers should advise patients to strictly adhere to recommended instructions for use. (2.2, 5.2, 17)
INDICATIONS AND USAGE
Testosterone gel 1.62% is indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired). (1)
- Hypogonadotropic hypogonadism (congenital or acquired). (1)
Limitations of use:
- Safety and efficacy of testosterone gel 1.62% in men with “age-related hypogonadism” have not been established. (1)
- Safety and efficacy of testosterone gel 1.62% in males less than 18 years old have not been established. (1, 8.4)
- Topical testosterone products may have different doses, strengths, or application instructions that may result in different systemic exposure. (1, 12.3)
DOSAGE AND ADMINISTRATION
-
Dosage and Administration for testosterone gel 1.62% differs from testosterone gel 1%. For dosage and administration of testosterone gel 1% refer to its full prescribing information. (2)
- Prior to initiating testosterone gel 1.62%, confirm the diagnosis of hypogonadism by ensuring that serum testosterone has been measured in the morning on at least two separate days and that these concentrations are below the normal range (2).
- Starting dose of testosterone gel 1.62% is 40.5 mg of testosterone (2 pump actuations or a single 40.5 mg packet), applied topically once daily in the morning. (2.1)
- Apply to clean, dry, intact skin of the shoulders and upper arms. Do not apply testosterone gel 1.62% to any other parts of the body including the abdomen, genitals, chest, armpits (axillae), or knees. (2.2, 12.3)
- Dose adjustment: testosterone gel 1.62% can be dose adjusted between a minimum of 20.25 mg of testosterone (1 pump actuation or a single 20.25 mg packet) and a maximum of 81 mg of testosterone (4 pump actuations or two 40.5 mg packets). The dose should be titrated based on the pre-dose morning serum testosterone concentration at approximately 14 days and 28 days after starting treatment or following dose adjustment. Additionally, serum testosterone concentration should be assessed periodically thereafter. (2.1)
- Patients should wash hands immediately with soap and water after applying testosterone gel 1.62% and cover the application site(s) with clothing after the gel has dried. Wash the application site thoroughly with soap and water prior to any situation where skin-to-skin contact of the application site with another person is anticipated. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Monitor patients with benign prostatic hyperplasia (BPH) for worsening of signs and symptoms of BPH. (5.1)
- Avoid unintentional exposure of women or children to testosterone gel 1.62%. Secondary exposure to testosterone can produce signs of virilization. Testosterone gel 1.62% should be discontinued until the cause of virilization is identified. (5.2)
- Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone products. Evaluate patients with signs or symptoms consistent with DVT or PE. (5.4)
- Some postmarketing studies have shown an increased risk of myocardial infarction and stroke associated with use of testosterone replacement therapy. (5.5)
- Exogenous administration of androgens may lead to azoospermia. (5.8)
- Edema with or without congestive heart failure (CHF) may be a complication in patients with preexisting cardiac, renal, or hepatic disease. (5.10)
- Sleep apnea may occur in those with risk factors. (5.12)
- Monitor serum testosterone, prostate specific antigen (PSA), hemoglobin, hematocrit, liver function tests and lipid concentrations periodically. (5.1, 5.3, 5.9, 5.13)
- Testosterone gel 1.62% is flammable until dry. (5.16)
ADVERSE REACTIONS
The most common adverse reaction (incidence ≥5%) is an increase in prostate specific antigen (PSA). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Androgens may decrease blood glucose and therefore may decrease insulin requirements in diabetic patients. (7.1)
- Changes in anticoagulant activity may be seen with androgens. More frequent monitoring of International Normalized Ratio (INR) and prothrombin time is recommended. (7.2)
- Use of testosterone with adrenocorticotrophic hormone (ACTH) or corticosteroids may result in increased fluid retention. Use with caution, particularly in patients with cardiac, renal, or hepatic disease. (7.3)
USE IN SPECIFIC POPULATIONS
There are insufficient long-term safety data in geriatric patients using testosterone gel 1.62% to assess the potential risks of cardiovascular disease and prostate cancer. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Virilization has been reported in children who were secondarily exposed to testosterone gel. (5.2, 6.2)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SECONDARY EXPOSURE TO TESTOSTERONE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Dose Adjustment
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
5.2 Potential for Secondary Exposure to Testosterone
5.3 Polycythemia
5.4 Venous Thromboembolism
5.5 Cardiovascular Risk
5.6 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
5.7 Use in Women
5.8 Potential for Adverse Effects on Spermatogenesis
5.9 Hepatic Adverse Effects
5.10 Edema
5.11 Gynecomastia
5.12 Sleep Apnea
5.13 Lipids
5.14 Hypercalcemia
5.15 Decreased Thyroxine-binding Globulin
5.16 Flammability
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Insulin
7.2 Oral Anticoagulants
7.3 Corticosteroids
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials in Hypogonadal Males
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Use in Men with Known or Suspected Prostate or Breast Cancer
17.2 Potential for Secondary Exposure to Testosterone and Steps to Prevent Secondary Exposure
17.3 Potential Adverse Reactions with Androgens
17.4 Patients Should Be Advised of the Following Instructions for Use
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SECONDARY EXPOSURE TO TESTOSTERONE
-
Virilization has been reported in children who were secondarily exposed to testosterone gel [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
-
Children should avoid contact with unwashed or unclothed application sites in men using testosterone gel [see Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
- Healthcare providers should advise patients to strictly adhere to recommended instructions for use [see Dosage and Administration (2.2), Warnings and Precautions (5.2) and Patient Counseling Information (17)].
-
Virilization has been reported in children who were secondarily exposed to testosterone gel [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
-
1 INDICATIONS AND USAGE
Testosterone gel 1.62% is indicated for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
• Primary hypogonadism (congenital or acquired): testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
• Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations, but have gonadotropins in the normal or low range.
Limitations of use:
• Safety and efficacy of testosterone gel 1.62% in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
• Safety and efficacy of testosterone gel 1.62% in males less than 18 years old have not been established [see Use in Specific Populations (8.4)].
• Topical testosterone products may have different doses, strengths, or application instructions that may result in different systemic exposure [see Indications and Usage (1), and Clinical Pharmacology (12.3)].
-
2 DOSAGE AND ADMINISTRATION
Dosage and Administration for testosterone gel 1.62% differs from testosterone gel 1%. For dosage and administration of testosterone gel 1% refer to its full prescribing information. (2)
Prior to initiating testosterone gel 1.62%, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
2.1 Dosing and Dose Adjustment
The recommended starting dose of testosterone gel 1.62% is 40.5 mg of testosterone (2 pump actuations or a single 40.5 mg packet) applied topically once daily in the morning to the shoulders and upper arms.
The dose can be adjusted between a minimum of 20.25 mg of testosterone (1 pump actuation or a single 20.25 mg packet) and a maximum of 81 mg of testosterone (4 pump actuations or two 40.5 mg packets). To ensure proper dosing, the dose should be titrated based on the pre-dose morning serum testosterone concentration from a single blood draw at approximately 14 days and 28 days after starting treatment or following dose adjustment. In addition, serum testosterone concentration should be assessed periodically thereafter. Table 1 describes the dose adjustments required at each titration step.
Table 1: Dose Adjustment Criteria Pre-Dose Morning Total Serum Testosterone Concentration Dose Titration Greater than 750 ng/dL Decrease daily dose by 20.25 mg (1 pump actuation or the equivalent of one 20.25 mg packet) Equal to or greater than 350 and equal to
or less than 750 ng/dLNo change: continue on current dose Less than 350 ng/dL Increase daily dose by 20.25 mg (1 pump actuation or the equivalent of one 20.25 mg packet) The application site and dose of testosterone gel 1.62% are not interchangeable with other topical testosterone products.
2.2 Administration Instructions
Testosterone gel 1.62% should be applied to clean, dry, intact skin of the upper arms and shoulders. Do not apply testosterone gel 1.62% to any other parts of the body, including the abdomen, genitals, chest, armpits (axillae), or knees [see Clinical Pharmacology (12.3)]. Area of application should be limited to the area that will be covered by the patient's short sleeve t-shirt. Patients should be instructed to use the palm of the hand to apply testosterone gel 1.62% and spread across the maximum surface area as directed in Table 2 (for pump) and Table 3 (for packets) and in Figure 1.
Table 2: Application Sites for Testosterone Gel 1.62%, Pump Total Dose of Testosterone Total Pump Actuations Pump Actuations Per Upper Arm and Shoulder Upper Arm and Shoulder #1 Upper Arm and Shoulder #2 20.25 mg 1 1 0 40.5 mg 2 1 1 60.75 mg 3 2 1 81 mg 4 2 2 Table 3: Application Sites for Testosterone Gel 1.62%, Packets Total Dose of Testosterone
Total packets
Gel Applications Per Upper Arm and Shoulder
Upper Arm and Shoulder #1
Upper Arm and Shoulder #2
20.25 mg One 20.25 mg packet One 20.25 mg packet 0 40.5 mg
One 40.5 mg packet
Half of contents of One 40.5 mg packet
Half of contents of One 40.5 mg packet
60.75 mg One 20.25 mg packet and One 40.5 mg packet One 40.5 mg packet One 20.25 mg packet 81 mg
Two 40.5 mg packets
One 40.5 mg packet
One 40.5 mg packet
The prescribed daily dose of testosterone gel 1.62% should be applied to the right and left upper arms and shoulders as shown in the shaded areas in Figure 1.
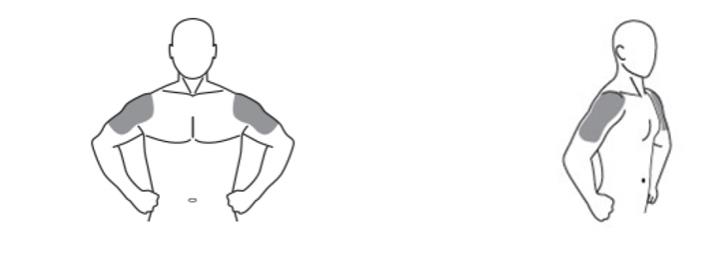
Figure 1. Application Sites for Testosterone Gel 1.62%
Once the application site is dry, the site should be covered with clothing [see Clinical Pharmacology (12.3)]. Wash hands thoroughly with soap and water. Avoid fire, flames or smoking until the gel has dried since alcohol based products, including testosterone gel 1.62%, are flammable.
The patient should avoid swimming or showering or washing the administration site for a minimum of 2 hours after application [see Clinical Pharmacology (12.3)].
To obtain a full first dose, it is necessary to prime the canister pump. To do so, with the canister in the upright position, slowly and fully depress the actuator three times. Safely discard the gel from the first three actuations. It is only necessary to prime the pump before the first dose.
After the priming procedure, fully depress the actuator once for every 20.25 mg of testosterone gel 1.62%. Testosterone gel 1.62% should be delivered directly into the palm of the hand and then applied to the application sites.
When using packets, the entire contents should be squeezed into the palm of the hand and immediately applied to the application sites. When 40.5 mg packets need to be split between the left and right shoulder, patients may squeeze a portion of the gel from the packet into the palm of the hand and apply to application sites. Repeat until entire contents have been applied. Alternatively, Testosterone gel 1.62% can be applied directly to the application sites from the pump or packets.
Strict adherence to the following precautions is advised in order to minimize the potential for secondary exposure to testosterone from testosterone gel 1.62%-treated skin:
- Children and women should avoid contact with unwashed or unclothed application site(s) of men using testosterone gel 1.62%.
- Testosterone gel 1.62% should only be applied to the upper arms and shoulders. The area of application should be limited to the area that will be covered by a short sleeve t-shirt.
- Patients should wash their hands with soap and water immediately after applying testosterone gel 1.62%.
- Patients should cover the application site(s) with clothing (e.g., a t-shirt) after the gel has dried.
- Prior to situations in which direct skin-to-skin contact is anticipated, patients should wash the application site(s) thoroughly with soap and water to remove any testosterone residue.
- In the event that unwashed or unclothed skin to which testosterone gel 1.62% has been applied comes in direct contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible.
- Children and women should avoid contact with unwashed or unclothed application site(s) of men using testosterone gel 1.62%.
-
3 DOSAGE FORMS AND STRENGTHS
Testosterone gel 1.62% for topical use only, is available as follows:
- A metered-dose pump. Each pump actuation delivers 20.25 mg of testosterone in 1.25 g of gel.
- A unit dose packet containing 20.25 mg of testosterone in 1.25 g of gel.
- A unit dose packet containing 40.5 mg of testosterone in 2.5 g of gel.
-
4 CONTRAINDICATIONS
- Testosterone gel 1.62% is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- Testosterone gel 1.62% is contraindicated in women who are pregnant. Testosterone gel 1.62% can cause virilization of the female fetus when administered to a pregnant woman. Pregnant women need to be aware of the potential for transfer of testosterone from men treated with testosterone gel 1.62%. If a pregnant woman is exposed to testosterone gel 1.62%, she should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
- Testosterone gel 1.62% is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
- Patients with BPH treated with androgens are at an increased risk for worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
- Patients treated with androgens may be at increased risk for prostate cancer. Evaluation of patients for prostate cancer prior to initiating and during treatment with androgens is appropriate [see Contraindications (4)].
5.2 Potential for Secondary Exposure to Testosterone
Cases of secondary exposure resulting in virilization of children have been reported in postmarketing surveillance of testosterone gel products. Signs and symptoms have included enlargement of the penis or clitoris, development of pubic hair, increased erections and libido, aggressive behavior, and advanced bone age. In most cases, these signs and symptoms regressed with removal of the exposure to testosterone gel. In a few cases, however, enlarged genitalia did not fully return to age-appropriate normal size, and bone age remained modestly greater than chronological age. The risk of transfer was increased in some of these cases by not adhering to precautions for the appropriate use of the topical testosterone product. Children and women should avoid contact with unwashed or unclothed application sites in men using testosterone gel 1.62% [see Dosage and Administration (2.2), Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
Inappropriate changes in genital size or development of pubic hair or libido in children, or changes in body hair distribution, significant increase in acne, or other signs of virilization in adult women should be brought to the attention of a physician and the possibility of secondary exposure to testosterone gel should also be brought to the attention of a physician. Testosterone gel should be promptly discontinued until the cause of virilization has been identified.
5.3 Polycythemia
Increases in hematocrit, reflective of increases in red blood cell mass, may require lowering or discontinuation of testosterone. Check hematocrit prior to initiating treatment. It would also be appropriate to re-evaluate the hematocrit 3 to 6 months after starting treatment, and then annually. If hematocrit becomes elevated, stop therapy until hematocrit decreases to an acceptable concentration. An increase in red blood cell mass may increase the risk of thromboembolic events.
5.4 Venous Thromboembolism
There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products such as testosterone gel 1.62%. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with testosterone gel 1.62% and initiate appropriate workup and management [see Adverse Reactions (6.2)].
5.5 Cardiovascular Risk
Long term clinical safety trials have not been conducted to assess the cardiovascular outcomes of testosterone replacement therapy in men. To date, epidemiologic studies and randomized controlled trials have been inconclusive for determining the risk of major adverse cardiovascular events (MACE), such as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, with the use of testosterone compared to non-use. Some studies, but not all, have reported an increased risk of MACE in association with use of testosterone replacement therapy in men. Patients should be informed of this possible risk when deciding whether to use or to continue to use testosterone gel 1.62%.
5.6 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Anabolic androgenic steroid abuse can lead to serious cardiovascular and psychiatric adverse reactions [see Drug Abuse and Dependence (9)].
If testosterone abuse is suspected, check serum testosterone concentrations to ensure they are within therapeutic range. However, testosterone levels may be in the normal or subnormal range in men abusing synthetic testosterone derivatives. Counsel patients concerning the serious adverse reactions associated with abuse of testosterone and anabolic androgenic steroids. Conversely, consider the possibility of testosterone and anabolic androgenic steroid abuse in suspected patients who present with serious cardiovascular or psychiatric adverse events.
5.7 Use in Women
Due to the lack of controlled evaluations in women and potential virilizing effects, testosterone gel 1.62% is not indicated for use in women [see Contraindications (4) and Use in Specific Populations (8.1, 8.2)].
5.8 Potential for Adverse Effects on Spermatogenesis
With large doses of exogenous androgens, including testosterone gel 1.62%, spermatogenesis may be suppressed through feedback inhibition of pituitary FSH possibly leading to adverse effects on semen parameters including sperm count.
5.9 Hepatic Adverse Effects
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. Testosterone gel 1.62% is not known to cause these adverse effects.
5.10 Edema
Androgens, including testosterone gel 1.62%, may promote retention of sodium and water. Edema, with or without congestive heart failure, may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease [see Adverse Reactions (6.2)].
5.11 Gynecomastia
Gynecomastia may develop and persist in patients being treated with androgens, including testosterone gel 1.62%, for hypogonadism.
5.12 Sleep Apnea
The treatment of hypogonadal men with testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung diseases.
5.13 Lipids
Changes in serum lipid profile may require dose adjustment or discontinuation of testosterone therapy.
5.14 Hypercalcemia
Androgens, including testosterone gel 1.62 %, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Regular monitoring of serum calcium concentrations is recommended in these patients.
5.15 Decreased Thyroxine-binding Globulin
Androgens, including testosterone gel 1.62%, may decrease concentrations of thyroxin-binding globulins, resulting in decreased total T4 serum concentrations and increased resin uptake of T3 and T4. Free thyroid hormone concentrations remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
- Patients with BPH treated with androgens are at an increased risk for worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Testosterone gel 1.62% was evaluated in a two-phase, 364-day, controlled clinical study. The first phase was a multi-center, randomized, double-blind, parallel-group, placebo-controlled period of 182 days, in which 234 hypogonadal men were treated with testosterone gel 1.62% and 40 received placebo. Patients could continue in an open-label, non-comparative, maintenance period for an additional 182 days [see Clinical Studies (14.1)].
The most common adverse reaction reported in the double-blind period was increased prostate specific antigen (PSA) reported in 26 testosterone gel 1.62%-treated patients (11.1%). In 17 patients, increased PSA was considered an adverse event by meeting one of the two pre-specified criteria for abnormal PSA values, defined as (1) average serum PSA >4 ng/mL based on two separate determinations, or (2) an average change from baseline in serum PSA of greater than 0.75 ng/mL on two determinations.
During the 182-day, double-blind period of the clinical trial, the mean change in serum PSA value was 0.14 ng/mL for patients receiving testosterone gel 1.62% and -0.12 ng/mL for the patients in the placebo group. During the double-blind period, seven patients had a PSA value >4.0 ng/mL, four of these seven patients had PSA less than or equal to 4.0 ng/mL upon repeat testing. The other three patients did not undergo repeat PSA testing.
During the 182-day, open-label period of the study, the mean change in serum PSA values was 0.10 ng/mL for both patients continuing on active therapy and patients transitioning onto active from placebo. During the open-label period, three patients had a serum PSA value > 4.0 ng/mL, two of whom had a serum PSA less than or equal to 4.0 ng/mL upon repeated testing. The other patient did not undergo repeat PSA testing. Among previous placebo patients, 3 of 28 (10.7%), had increased PSA as an adverse event in the open-label period.
Table 4 shows adverse reactions reported by > 2% of patients in the 182-day, double-blind period of the testosterone gel 1.62% clinical trial and more frequent in the testosterone gel 1.62% treated group versus placebo.
Table 4: Adverse Reactions Reported in >2% of Patients in the 182-Day, Double-Blind Period of Testosterone Gel 1.62% Clinical Trial Number (%) of Patients Adverse Reaction Testosterone Gel 1.62%
N=234Placebo
N=40PSA increased* 26 (11.1%) 0% Emotional lability** 6 (2.6%) 0% Hypertension 5 (2.1%) 0% Hematocrit or hemoglobin increased 5 (2.1%) 0% Contact dermatitis*** 5 (2.1%) 0% *PSA increased includes: PSA values that met pre-specified criteria for abnormal PSA values (an average change from baseline > 0.75 ng/mL and/or an average PSA value >4.0 ng/mL based on two measurements) as well as those reported as adverse events. **Emotional lability includes: mood swings, affective disorder, impatience, anger, and aggression. ***Contact dermatitis includes: 4 patients with dermatitis at non-application sites. Other adverse reactions occurring in less than or equal to 2% of testosterone gel 1.62%-treated patients and more frequently than placebo included: frequent urination, and hyperlipidemia.
In the open-label period of the study (N=191), the most commonly reported adverse reaction (experienced by greater than 2% of patients) was increased PSA (n=13; 6.2%) and sinusitis. Other adverse reactions reported by less than or equal to 2% of patients included increased hemoglobin or hematocrit, hypertension, acne, libido decreased, insomnia, and benign prostatic hypertrophy.
During the 182-day, double-blind period of the clinical trial, 25 testosterone gel 1.62%-treated patients (10.7%) discontinued treatment because of adverse reactions. These adverse reactions included 17 patients with PSA increased and 1 report each of: hematocrit increased, blood pressure increased, frequent urination, diarrhea, fatigue, pituitary tumor, dizziness, skin erythema and skin nodule (same patient – neither at application site), vasovagal syncope, and diabetes mellitus. During the 182-day, open-label period, 9 patients discontinued treatment because of adverse reactions. These adverse reactions included 6 reports of PSA increased, 2 of hematocrit increased, and 1 each of triglycerides increased and prostate cancer.
Application Site Reactions
In the 182-day double-blind period of the study, application site reactions were reported in two (2/234; 0.9%) patients receiving testosterone gel 1.62%, both of which resolved. Neither of these patients discontinued the study due to application site adverse reactions. In the open-label period of the study, application site reactions were reported in three (3/219; 1.4%) additional patients that were treated with testosterone gel 1.62%. None of these subjects were discontinued from the study due to application site reactions.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of testosterone gel 1%. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure (Table 5).
Table 5: Adverse Reactions from Post Approval Experience of Testosterone Gel 1% by System Organ Class System Organ Class Adverse Reaction Blood and lymphatic system disorders: Elevated hemoglobin or hematocrit, polycythemia, anemia Cardiovascular disorders: Myocardial infarction, stroke Endocrine disorders: Hirsutism Gastrointestinal disorders: Nausea General disorders: Asthenia, edema, malaise Genitourinary disorders: Impaired urination* Hepatobiliary disorders: Abnormal liver function tests Investigations: Lab test abnormal**, elevated PSA, electrolyte changes (nitrogen, calcium, potassium [includes hypokalemia], phosphorus, sodium), impaired glucose tolerance, hyperlipidemia, HDL, fluctuating testosterone levels, weight increase Neoplasms: Prostate cancer Nervous system disorders: Dizziness, headache, insomnia, sleep apnea Psychiatric disorders: Amnesia, anxiety, depression, hostility, emotional lability, decreased libido, nervousness Reproductive system and breast disorders: Gynecomastia, mastodynia, oligospermia, priapism (frequent or prolonged erections), prostate enlargement, BPH, testis disorder*** Respiratory disorders: Dyspnea Skin and subcutaneous tissue disorders: Acne, alopecia, application site reaction (discolored hair, dry skin, erythema, paresthesia, pruritus, rash), skin dry, pruritus, sweating Vascular disorders: Hypertension, vasodilation (hot flushes), venous thromboembolism * Impaired urination includes nocturia, urinary hesitancy, urinary incontinence, urinary retention, urinary urgency and weak urinary stream **Lab test abnormal includes elevated AST, elevated ALT, elevated testosterone, elevated hemoglobin or hematocrit, elevated cholesterol, elevated cholesterol/LDL ratio, elevated triglycerides, or elevated serum creatinine ***Testis disorder includes atrophy or non-palpable testis, varicocele, testis sensitivity or tenderness Secondary Exposure to Testosterone in Children
Cases of secondary exposure to testosterone resulting in virilization of children have been reported in postmarketing surveillance of testosterone gel products. Signs and symptoms of these reported cases have included enlargement of the clitoris (with surgical intervention) or the penis, development of pubic hair, increased erections and libido, aggressive behavior, and advanced bone age. In most cases with a reported outcome, these signs and symptoms were reported to have regressed with removal of the testosterone gel exposure. In a few cases, however, enlarged genitalia did not fully return to age appropriate normal size, and bone age remained modestly greater than chronological age. In some of the cases, direct contact with the sites of application on the skin of men using testosterone gel was reported. In at least one reported case, the reporter considered the possibility of secondary exposure from items such as the testosterone gel user's shirts and/or other fabric, such as towels and sheets [see Warnings and Precautions (5.2)].
-
7 DRUG INTERACTIONS
7.1 Insulin
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, may decrease insulin requirements.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Testosterone gel 1.62% is contraindicated in pregnant women. Testosterone is teratogenic and may cause fetal harm when administered to a pregnant woman based on data from animal studies and its mechanism of action [see Contraindications (4) and Clinical Pharmacology (12.1)]. Exposure of a female fetus to androgens may result in varying degrees of virilization. In animal developmental studies, exposure to testosterone in utero resulted in hormonal and behavioral changes in offspring and structural impairments of reproductive tissues in female and male offspring. These studies did not meet current standards for nonclinical development toxicity studies.
Data
Animal Data
In developmental studies conducted in rats, rabbits, pigs, sheep and rhesus monkeys, pregnant animals received intramuscular injection of testosterone during the period of organogenesis. Testosterone treatment at doses that were comparable to those used for testosterone replacement therapy resulted in structural impairments in both female and male offspring. Structural impairments observed in females included increased ano-genital distance, phallus development, empty scrotum, no external vagina, intrauterine growth retardation, reduced ovarian reserve, and increased ovarian follicular recruitment. Structural impairments seen in male offspring included increased testicular weight, larger seminal tubular lumen diameter, and higher frequency of occluded tubule lumen. Increased pituitary weight was seen in both sexes.
Testosterone exposure in utero also resulted in hormonal and behavioral changes in offspring. Hypertension was observed in pregnant female rats and their offspring exposed to doses approximately twice those used for testosterone replacement therapy.
8.3 Females and Males of Reproductive Potential
Infertility
Testis disorder, testicular atrophy, and oligospermia have been identified during use of testosterone gel 1.62% [see Adverse Reactions (6.1, 6.2)].
During treatment with large doses of exogenous androgens, including testosterone gel 1.62%, spermatogenesis may be suppressed through feedback inhibition of the hypothalamic-pituitary-testicular axis [see Warnings and Precautions (5.8)]. Reduced fertility is observed in some men taking testosterone replacement therapy. Testicular atrophy, subfertility, and infertility have also been reported in men who abuse anabolic androgenic steroids [see Drug Abuse and Dependence (9.2)]. With either type of use, the impact on fertility may be irreversible.
8.4 Pediatric Use
The safety and effectiveness of testosterone gel 1.62% in pediatric patients less than 18 years old has not been established. Improper use may result in acceleration of bone age and premature closure of epiphyses.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric patients involved in controlled clinical studies utilizing testosterone gel 1.62% to determine whether efficacy in those over 65 years of age differs from younger subjects. Of the 234 patients enrolled in the clinical trial utilizing testosterone gel 1.62%, 21 were over 65 years of age. Additionally, there is insufficient long-term safety data in geriatric patients to assess the potentially increased risks of cardiovascular disease and prostate cancer.
Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of BPH.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Testosterone gel 1.62% contains testosterone, a Schedule III controlled substance in the Controlled Substances Act.
9.2 Abuse
Drug abuse is intentional non-therapeutic use of a drug, even once, for its rewarding psychological and physiological effects. Abuse and misuse of testosterone are seen in male and female adults and adolescents. Testosterone, often in combination with other anabolic androgenic steroids (AAS), and not obtained by prescription through a pharmacy, may be abused by athletes and bodybuilders. There have been reports of misuse by men taking higher doses of legally obtained testosterone than prescribed and continuing testosterone despite adverse events or against medical advice.
Abuse-Related Adverse Reactions
Serious adverse reactions have been reported in individuals who abuse anabolic androgenic steroids and include cardiac arrest, myocardial infarction, hypertrophic cardiomyopathy, congestive heart failure, cerebrovascular accident, hepatotoxicity, and serious psychiatric manifestations, including major depression, mania, paranoia, psychosis, delusions, hallucinations, hostility and aggression.
The following adverse reactions have also been reported in men: transient ischemic attacks, convulsions, hypomania, irritability, dyslipidemias, testicular atrophy, subfertility, and infertility.
The following additional adverse reactions have been reported in women: hirsutism, virilization, deepening of voice, clitoral enlargement, breast atrophy, male-pattern baldness, and menstrual irregularities.
The following adverse reactions have been reported in male and female adolescents: premature closure of bony epiphyses with termination of growth, and precocious puberty.
Because these reactions are reported voluntarily from a population of uncertain size and may include abuse of other agents, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
9.3 Dependence
Behaviors Associated with Addiction
Continued abuse of testosterone and other anabolic steroids, leading to addiction is characterized by the following behaviors:
- Taking greater dosages than prescribed
- Continued drug use despite medical and social problems due to drug use
- Spending significant time to obtain the drug when supplies of the drug are interrupted
- Giving a higher priority to drug use than other obligations
- Having difficulty in discontinuing the drug despite desires and attempts to do so
- Experiencing withdrawal symptoms upon abrupt discontinuation of use
Physical dependence is characterized by withdrawal symptoms after abrupt drug discontinuation or a significant dose reduction of a drug. Individuals taking supratherapeutic doses of testosterone may experience withdrawal symptoms lasting for weeks or months which include depressed mood, major depression, fatigue, craving, restlessness, irritability, anorexia, insomnia, decreased libido and hypogonadotropic hypogonadism.
Drug dependence in individuals using approved doses of testosterone for approved indications has not been documented.
-
10 OVERDOSAGE
There is a single report of acute overdosage after parenteral administration of an approved testosterone product in the literature. This subject had serum testosterone concentrations of up to 11,400 ng/dL, which were implicated in a cerebrovascular accident. There were no reports of overdosage in the testosterone gel 1.62% clinical trial.
Treatment of overdosage would consist of discontinuation of testosterone gel 1.62%, washing the application site with soap and water, and appropriate symptomatic and supportive care.
-
11 DESCRIPTION
Testosterone gel 1.62% for topical use is a clear, colorless gel containing testosterone, USP. Testosterone is an androgen. Testosterone gel 1.62% is available in a metered-dose pump or unit dose packets.
The active pharmacologic ingredient in testosterone gel 1.62% is testosterone. Testosterone, USP is a white to almost white powder chemically described as 17-beta hydroxyandrost-4-en-3-one. The structural formula is:
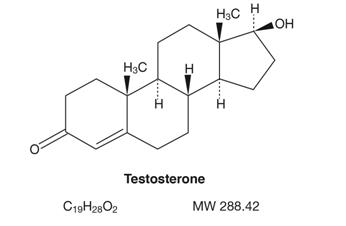
The inactive ingredients in testosterone gel 1.62% are: carbomer homopolymer type C (carbopol 980), ethyl alcohol, isopropyl myristate, purified water, and sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as facial, pubic, chest and axillary hair; laryngeal enlargement; vocal chord thickening; and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter's syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).
12.2 Pharmacodynamics
No specific pharmacodynamic studies were conducted using testosterone gel 1.62%.
12.3 Pharmacokinetics
Absorption
Testosterone gel 1.62% delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate normal levels (300 ng/dL to 1000 ng/dL) seen in healthy men. Testosterone gel 1.62% provides continuous transdermal delivery of testosterone for 24 hours following once daily application to clean, dry, intact skin of the shoulders and upper arms. Average serum testosterone concentrations over 24 hours (Cavg) observed when testosterone gel 1.62% was applied to the upper arms/shoulders were comparable to average serum testosterone concentrations (Cavg) when testosterone gel 1.62% was applied using a rotation method utilizing the abdomen and upper arms/shoulders. The rotation of abdomen and upper arms/shoulders was a method used in the pivotal clinical trial [see Clinical Studies (14.1)].
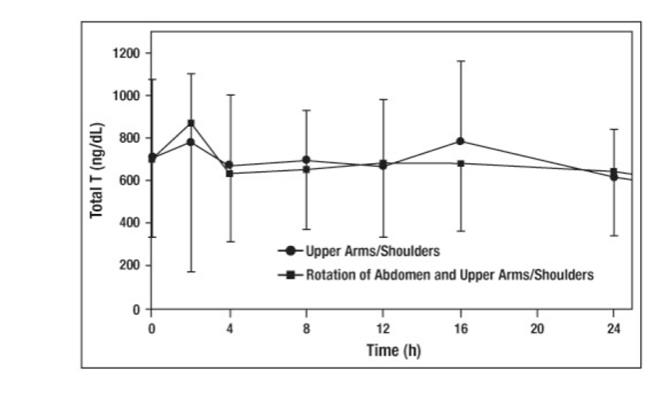
Figure 2: Mean (±SD) Serum Total Testosterone Concentrations on Day 7 in Patients Following Testosterone Gel 1.62% Once-Daily Application of 81 mg of Testosterone (N=33) for 7 Days
Distribution
Circulating testosterone is primarily bound in the serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is loosely bound to albumin and other proteins.
Metabolism
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and DHT.
Excretion
There is considerable variation in the half-life of testosterone concentration as reported in the literature, ranging from 10 to 100 minutes. About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic acid and sulfuric acid conjugates of testosterone and its metabolites. About 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
When testosterone gel 1.62% treatment is discontinued, serum testosterone concentrations return to approximately baseline concentrations within 48 to 72 hours after administration of the last dose.
Potential for testosterone transfer
The potential for testosterone transfer following administration of testosterone gel 1.62% when it was applied only to upper arms/shoulders was evaluated in two clinical studies of males dosed with testosterone gel 1.62% and their untreated female partners. In one study, 8 male subjects applied a single dose of testosterone gel 1.62% 81 mg to their shoulders and upper arms. Two (2) hours after application, female subjects rubbed their hands, wrists, arms, and shoulders to the application site of the male subjects for 15 minutes. Serum concentrations of testosterone were monitored in female subjects for 24 hours after contact occurred. After direct skin-to-skin contact with the site of application, mean testosterone Cavg and Cmax in female subjects increased by 280% and 267%, respectively, compared to mean baseline testosterone concentrations. In a second study evaluating transfer of testosterone, 12 male subjects applied a single dose of testosterone gel 1.62% 81 mg to their shoulders and upper arms. Two (2) hours after application, female subjects rubbed their hands, wrists, arms, and shoulders to the application site of the male subjects for 15 minutes while the site of application was covered by a t-shirt. When a t-shirt was used to cover the site of application, mean testosterone Cavg and Cmax in female subjects increased by 6% and 11%, respectively, compared to mean baseline testosterone concentrations.
A separate study was conducted to evaluate the potential for testosterone transfer from 16 males dosed with testosterone gel 1.62% 81 mg when it was applied to abdomen only for 7 days, a site of application not approved for testosterone gel 1.62%. Two (2) hours after application to the males on each day, the female subjects rubbed their abdomens for 15 minutes to the abdomen of the males. The males had covered the application area with a T-shirt. The mean testosterone Cavg and Cmax in female subjects on day 1 increased by 43% and 47%, respectively, compared to mean baseline testosterone concentrations. The mean testosterone Cavg and Cmax in female subjects on day 7 increased by 60% and 58%, respectively, compared to mean baseline testosterone concentrations.
Effect of showering
In a randomized, 3-way (3 treatment periods without washout period) crossover study in 24 hypogonadal men, the effect of showering on testosterone exposure was assessed after once daily application of testosterone gel 1.62% 81 mg to upper arms/shoulders for 7 days in each treatment period. On the 7th day of each treatment period, hypogonadal men took a shower with soap and water at either 2, 6, or 10 hours after drug application. The effect of showering at 2 or 6 hours post-dose on Day 7 resulted in 13% and 12% decreases in mean Cavg, respectively, compared to Day 6 when no shower was taken after drug application. Showering at 10 hours after drug application had no effect on bioavailability. The amount of testosterone remaining in the outer layers of the skin at the application site on the 7th day was assessed using a tape stripping procedure and was reduced by at least 80% after showering 2 to 10 hours post-dose compared to on the 6th day when no shower was taken after drug application.
Effect of hand washing
In a randomized, open-label, single-dose, 2-way crossover study in 16 healthy male subjects, the effect of hand washing on the amount of residual testosterone on the hands was evaluated. Subjects used their hands to apply the maximum dose (81 mg testosterone) of testosterone gel 1.62% to their upper arms and shoulders. Within 1 minute of applying the gel, subjects either washed or did not wash their hands prior to study personnel wiping the subjects’ hands with ethanol dampened gauze pads. The gauze pads were then analyzed for residual testosterone content. A mean (SD) of 0.1 (0.04) mg of residual testosterone (0.12% of the actual applied dose of testosterone, and a 96% reduction compared to when hands were not washed) was recovered after washing hands with water and soap.
Effect of sunscreen or moisturizing lotion on absorption of testosterone
In a randomized, 3-way (3 treatment periods without washout period) crossover study in 18 hypogonadal males, the effect of applying a moisturizing lotion or a sunscreen on the absorption of testosterone was evaluated with the upper arms/shoulders as application sites. For 7 days, moisturizing lotion or sunscreen (SPF 50) was applied daily to the testosterone gel 1.62% application site 1 hour after the application of testosterone gel 1.62% 40.5 mg. Application of moisturizing lotion increased mean testosterone Cavg and Cmax by 14% and 17%, respectively, compared to testosterone gel 1.62% administered alone. Application of sunscreen increased mean testosterone Cavg and Cmax by 8% and 13%, respectively, compared to testosterone gel 1.62% applied alone.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Mutagenesis
Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays.
Impairment of Fertility
The administration of exogenous testosterone has been reported to suppress spermatogenesis in rats, dogs, and non-human primates, which was reversible on cessation of the treatment.
-
14 CLINICAL STUDIES
14.1 Clinical Trials in Hypogonadal Males
Testosterone gel 1.62% was evaluated in a multi-center, randomized, double-blind, parallel-group, placebo-controlled study (182-day double-blind period) in 274 hypogonadal men with body mass index (BMI) 18 kg/m2 to 40 kg/m2 and 18 to 80 years of age (mean age 53.8 years). The patients had an average serum testosterone concentration of <300 ng/dL, as determined by two morning samples collected on the same visit. Patients were Caucasian 83%, Black 13%, Asian or Native American 4%. 7.5% of patients were Hispanic.
Patients were randomized to receive active treatment or placebo using a rotation method utilizing the abdomen and upper arms/shoulders for 182 days. All patients were started at a daily dose of 40.5 mg (two pump actuations) testosterone gel 1.62% or matching placebo on Day 1 of the study. Patients returned to the clinic on Day 14, Day 28, and Day 42 for predose serum total testosterone assessments. The patient's daily dose was titrated up or down in 20.25 mg increments if the predose serum testosterone value was outside the range of 350 ng/dL to 750 ng/dL. The study included four active testosterone gel 1.62% doses: 20.25 mg, 40.5 mg, 60.75 mg, and 81 mg daily.
The primary endpoint was the percentage of patients with Cavg within the normal range of 300 ng/dL to 1000 ng/dL on Day 112. In patients treated with testosterone gel 1.62%, 81.6% (146/179) had Cavg within the normal range at Day 112. The secondary endpoint was the percentage of patients, with Cmax above three pre-determined limits. The percentages of patients with Cmax greater than 1500 ng/dL, and between 1800 and 2499 ng/dL on Day 112 were 11.2% and 5.5%, respectively. Two patients had a Cmax >2500 ng/dL on Day 112 (2510 ng/dL and 2550 ng/dL, respectively); neither of these 2 patients demonstrated an abnormal Cmax on prior or subsequent assessments at the same dose.
Patients could agree to continue in an open-label, active treatment maintenance period of the study for an additional 182 days.
Dose titrations on Days 14, 28, and 42 resulted in final doses of 20.25 mg to 81 mg on Day 112 as shown in Table 6.
Table 6: Mean (SD) Testosterone Concentrations (Cavg and Cmax) by final dose on Days 112 and 364 Final Dose on Day 112 Parameter Placebo
(n=27)20.25 mg
(n=12)40.5 mg
(n=34)60.75 mg
(n=54)81 mg
(n=79)All Active
(n=179)Cavg (ng/dL) 303 (135) 457 (275) 524 (228) 643 (285) 537 (240) 561 (259) Cmax (ng/dL) 450 (349) 663 (473) 798 (439) 958 (497) 813 (479) 845 (480) Final Dose on Day 364 20.25 mg
(n=7)40.5 mg
(n=26)60.75 mg
(n=29)81 mg
(n=74)Continuing Active
(n=136)Cavg (ng/dL) 386 (130) 474 (176) 513 (222) 432 (186) 455 (192) Cmax (ng/dL) 562 (187) 715 (306) 839 (568) 649 (329) 697 (389) Figure 3 summarizes the pharmacokinetic profile of total testosterone in patients completing 112 days of testosterone gel 1.62% treatment administered as a starting dose of 40.5 mg of testosterone (2 pump actuations) for the initial 14 days followed by possible titration according to the follow-up testosterone measurements.
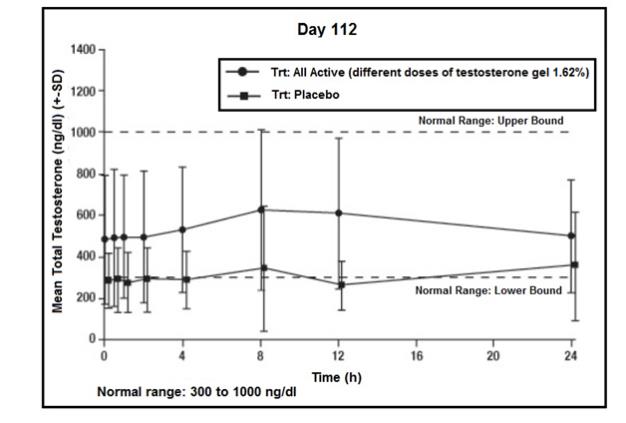
Figure 3: Mean (±SD) Steady-State Serum Total Testosterone Concentrations on Day 112
Efficacy was maintained in the group of men that received testosterone gel 1.62% for one full year. In that group, 78% (106/136) had average serum testosterone concentrations in the normal range at Day 364. Figure 4 summarizes the mean total testosterone profile for these patients on Day 364.
Figure 4: Mean (±SD) Steady-State Serum Total Testosterone Concentrations on Day 364
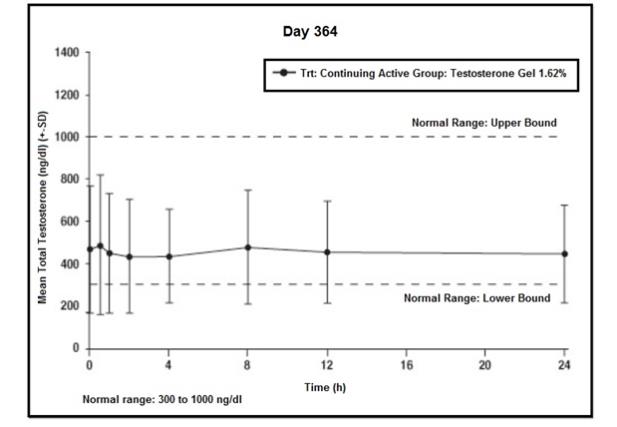
The mean estradiol and DHT concentration profiles paralleled the changes observed in testosterone. The levels of LH and FSH decreased with testosterone treatment. The decreases in levels of LH and FSH are consistent with reports published in the literature of long-term treatment with testosterone.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Testosterone gel 1.62% is supplied in non-aerosol, metered-dose pumps that deliver 20.25 mg of testosterone per complete pump actuation. The pumps are composed of plastic and stainless steel and an LDPE/aluminum foil inner liner encased in rigid plastic with a polypropylene cap. Each 88 g metered-dose pump is capable of dispensing 75 g of gel or 60-metered pump actuations; each pump actuation dispenses 1.25 g of gel.
Testosterone gel 1.62% is also supplied in unit-dose aluminum foil packets in cartons of 30. Each packet of 1.25 g or 2.5 g gel contains 20.25 mg or 40.5 mg testosterone, respectively.
NDC Number Package Size 0591-2924-18 88 g pump (each pump dispenses 60 metered pump actuations with each pump actuation containing 20.25 mg of testosterone in 1.25 g of gel) 0591-2925-32 Each unit dose packet contains 20.25 mg of testosterone provided in 1.25 g of gel 0591-2925-30 30 packets (each unit dose packet contains 20.25 mg of testosterone provided in 1.25 g of gel) 0591-2926-25 Each unit dose packet contains 40.5 mg of testosterone provided in 2.5 g of gel 0591-2926-30 30 packets (each unit dose packet contains 40.5 mg of testosterone provided in 2.5 g of gel) Store at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Used testosterone gel 1.62% pumps or used testosterone gel 1.62% packets should be discarded in household trash in a manner that prevents accidental application or ingestion by children or pets.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Medication Guide
Patients should be informed of the following:
17.1 Use in Men with Known or Suspected Prostate or Breast Cancer
Men with known or suspected prostate or breast cancer should not use testosterone gel 1.62% [see Contraindications (4) and Warnings and Precautions (5.1)].
17.2 Potential for Secondary Exposure to Testosterone and Steps to Prevent Secondary Exposure
Secondary exposure to testosterone in children and women can occur with the use of testosterone gel in men [see Warnings and Precautions (5.2)]. Cases of secondary exposure to testosterone have been reported in children.
Physicians should advise patients of the reported signs and symptoms of secondary exposure, which may include the following:
- In children: unexpected sexual development including inappropriate enlargement of the penis or clitoris, premature development of pubic hair, increased erections, and aggressive behavior.
- In women: changes in hair distribution, increase in acne, or other signs of testosterone effects.
- The possibility of secondary exposure to testosterone gel should be brought to the attention of a healthcare provider.
- Testosterone gel 1.62% should be promptly discontinued until the cause of virilization is identified.
Strict adherence to the following precautions is advised to minimize the potential for secondary exposure to testosterone from
testosterone gel 1.62% in men [see Medication Guide]:- Children and women should avoid contact with unwashed or unclothed application site(s) of men using testosterone gel 1.62%.
- Patients using testosterone gel 1.62% should apply the product as directed and strictly adhere to the following:
- Wash hands with soap and water immediately after application.
- Cover the application site(s) with clothing after the gel has dried.
- Wash the application site(s) thoroughly with soap and water prior to any situation where skin-to-skin contact of the application site with another person is anticipated.
- In the event that unwashed or unclothed skin to which testosterone gel 1.62% has been applied comes in contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible [see Dosage and Administration (2.2), Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
17.3 Potential Adverse Reactions with Androgens
Patients should be informed that treatment with androgens may lead to adverse reactions which include:
- Changes in urinary habits such as increased urination at night, trouble starting the urine stream, passing urine many times during the day, having an urge to go to the bathroom right away, having a urine accident, being unable to pass urine and weak urine flow.
- Breathing disturbances, including those associated with sleep, or excessive daytime sleepiness.
- Too frequent or persistent erections of the penis.
- Nausea, vomiting, changes in skin color, or ankle swelling.
17.4 Patients Should Be Advised of the Following Instructions for Use
- Read the Medication Guide before starting testosterone gel 1.62% therapy and to reread it each time the prescription is renewed.
- Testosterone gel 1.62% should be applied and used appropriately to maximize the benefits and to minimize the risk of secondary exposure in children and women.
- Keep testosterone gel 1.62% out of the reach of children.
- Testosterone gel 1.62% is an alcohol based product and is flammable; therefore avoid fire, flame or smoking until the gel has dried.
- It is important to adhere to all recommended monitoring.
- Report any changes in their state of health, such as changes in urinary habits, breathing, sleep, and mood.
- Testosterone gel 1.62% is prescribed to meet the patient's specific needs; therefore, the patient should never share testosterone gel 1.62% with anyone.
- Wait 2 hours before swimming or washing following application of testosterone gel 1.62%. This will ensure that the greatest amount of testosterone gel 1.62% is absorbed into their system.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Iss. 8/2019
-
Medication Guide
Testosterone (tes tosʹ ter one) Gel 1.62% CIII
for topical use
What is the most important information I should know about testosterone gel 1.62%?
1. Testosterone gel 1.62% can transfer from your body to others including, children and women. Children and women should avoid contact with the unwashed or not covered (unclothed) areas where testosterone gel 1.62% has been applied to your skin. Early signs and symptoms of puberty have occurred in young children who have come in direct contact with testosterone by touching areas where men have used testosterone gel 1.62%.
Children
Signs and symptoms of early puberty in a child when they come in direct contact with testosterone gel 1.62% may include:
Abnormal sexual changes:
- enlarged penis or clitoris.
- early growth of hair near the vagina or around the penis (pubic hair).
- erections or acting out sexual urges (sex drive).
Behavior problems:
- acting aggressively, behaving in an angry or violent way.
Women
Signs and symptoms in women when they come in direct contact with testosterone gel 1.62% may include:
- changes in body hair.
- an abnormal increase in pimples (acne).
Stop using testosterone gel 1.62% and call your healthcare provider right away if you see any signs and symptoms in a child or a woman that may have happened through accidental touching of the area where you have applied testosterone gel 1.62%.
2. To lower the risk of transfer of testosterone gel 1.62% from your body to others, follow these important instructions:
- Apply testosterone gel 1.62% only to your shoulders and upper arms that will be covered by a short sleeve t-shirt.
- Wash your hands right away with soap and water after applying testosterone gel 1.62%.
- After the gel has dried, cover the application area with clothing. Keep the area covered until you have washed the application area well or have showered.
- If you expect to have skin-to-skin contact with another person, first wash the application area well with soap and water.
- If a child or woman touches the area where you have applied testosterone gel 1.62%, that area on the child or woman should be washed well with soap and water right away.
What is testosterone gel 1.62%?
Testosterone gel 1.62% is a prescription medicine that contains testosterone. Testosterone gel 1.62% is used to treat adult males who have low or no testosterone due to certain medical conditions.
- Your healthcare provider will test your blood before you start and while you are using testosterone gel 1.62%.
- It is not known if testosterone gel 1.62% is safe or effective to treat men who have low testosterone due to aging.
- It is not known if testosterone gel 1.62% is safe or effective in children younger than 18 years old. Improper use of testosterone gel 1.62% may affect bone growth in children.
Testosterone gel 1.62% is a controlled substance (CIII) because it contains testosterone that can be a target for people who abuse prescription medicines. Keep your testosterone gel 1.62% in a safe place to protect it. Never give your testosterone gel 1.62% to anyone else, even if they have the same symptoms you have. Selling or giving away this medicine may harm others and is against the law.
Testosterone gel 1.62% is not meant for use in women.
Do not use testosterone gel 1.62% if you:
- have breast cancer.
- have or might have prostate cancer.
- are pregnant. Testosterone gel 1.62% may harm your unborn baby.
- Women who are pregnant should avoid contact with the area of skin where testosterone gel 1.62% has been applied.
Before using testosterone gel 1.62%, tell your healthcare provider about all of your medical conditions, including if you:
- have breast cancer.
- have or might have prostate cancer.
- have urinary problems due to an enlarged prostate.
- have heart problems.
- have kidney or liver problems.
- have problems breathing while you sleep (sleep apnea).
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using testosterone gel 1.62% with certain other medicines can affect each other.
Especially, tell your healthcare provider if you take:
- insulin
- medicines that decrease blood clotting (blood thinners)
- corticosteroids
How should I use testosterone gel 1.62%?
- See the detailed Instructions for Use about how to use testosterone gel 1.62% at the end of this Medication Guide.
- It is important that you apply testosterone gel 1.62% exactly as your healthcare provider tells you to.
- Your healthcare provider may change your testosterone gel 1.62% dose. Do not change your testosterone gel 1.62% dose without talking to your healthcare provider.
- Apply testosterone gel 1.62% at the same time each morning. Testosterone gel 1.62% should be applied after showering or bathing.
What are the possible side effects of testosterone gel 1.62%?
Testosterone gel 1.62% can cause serious side effects including:
See “What is the most important information I should know about testosterone gel 1.62%?”
- If you already have enlargement of your prostate gland your signs and symptoms can get worse while using testosterone gel 1.62%. This can include:
- increased urination at night.
- trouble starting your urine stream.
- having to pass urine many times during the day.
- having an urge to go to the bathroom right away.
- having a urine accident.
- being unable to pass urine or weak urine flow.
- Possible increased risk of prostate cancer. Your healthcare provider should check you for prostate cancer or any other prostate problems before you start and while you use testosterone gel 1.62%.
- Blood clots in the legs or lungs. Signs and symptoms of a blood clot in your leg can include leg pain, swelling, or redness. Signs and symptoms of a blood clot in your lungs can include difficulty breathing or chest pain.
- Possible increased risk of heart attack or stroke.
- In large doses testosterone gel 1.62% may lower your sperm count.
- Swelling of your ankles, feet, or body, with or without heart failure.
- Enlarged or painful breasts.
- Have problems breathing while you sleep (sleep apnea).
Call your healthcare provider right away if you have any of the serious side effects listed above.
The most common side effects of testosterone gel 1.62% include:
- increased prostate specific antigen (a test used to screen for prostate cancer)
- mood swings
- hypertension
- increased red blood cell count
- skin irritation where testosterone gel 1.62% is applied
Other side effects include more erections than are normal for you or erections that last a long time.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of testosterone gel 1.62%. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of testosterone gel 1.62%
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use testosterone gel 1.62% for a condition for which it was not prescribed. Do not give testosterone gel 1.62% to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about testosterone gel 1.62% that is written for health professionals.
What are the ingredients in testosterone gel 1.62%?
Active ingredient: testosterone
Inactive ingredients: carbomer homopolymer type C (carbopol 980), ethyl alcohol, isopropyl myristate, purified water and sodium hydroxide.
For more information, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. A 5/2019
-
INSTRUCTIONS FOR USE
Testosterone (tes tosʹ ter one) Gel 1.62% CIII
for topical use
Read this Instructions for Use for testosterone gel 1.62% before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Applying testosterone gel 1.62%:
Testosterone gel 1.62% comes in a pump or in packets.
- Before applying testosterone gel 1.62% make sure that your shoulders and upper arms are clean, dry, and that there is no broken skin.
- Testosterone gel 1.62% is to be applied to the area of your shoulders and upper arms that will be covered by a short sleeve t-shirt (See Figure A). Do not apply testosterone gel 1.62% to any other parts of your body such as your stomach area (abdomen), penis, scrotum, chest, armpits (axillae), or knees.
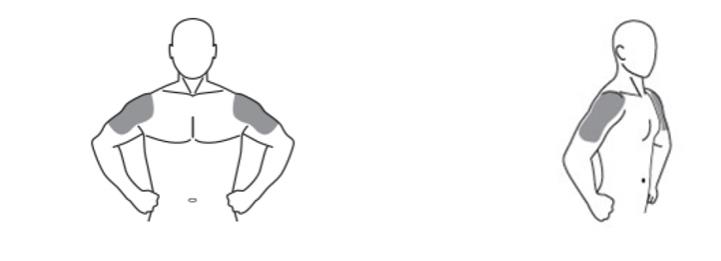
(Figure A)
If you are using testosterone gel 1.62% pump:
- Before using a new bottle of testosterone gel 1.62 % for the first time, you will need to remove the cap and then prime the pump. To prime the testosterone gel 1.62% pump, slowly push the pump all the way down 3 times, over the sink drain. Do not use any testosterone gel 1.62% that came out while priming. Wash it down the sink to avoid accidental exposure to others. Your testosterone gel 1.62% pump is now ready to use.
- Remove the cap from the pump. Then, put the spout opening at the top of the pump where the medicine comes out over the palm of your hand and slowly push the pump all the way down. Apply testosterone gel 1.62% to the application site. You may also apply testosterone gel 1.62% directly to the application site. Your healthcare provider will tell you the number of times to press the pump for each dose.
- Wash your hands with soap and water right away.
Find Your Dose as Prescribed by Your
Healthcare ProviderApplication Method 1 PUMP 20.25 mg Apply 1 pump of testosterone gel 1.62% to 1 upper arm and shoulder. 2 PUMPS 40.5 mg Apply 1 pump of testosterone gel 1.62% to 1 upper arm and shoulder and then apply 1 pump of testosterone gel 1.62% to the opposite upper arm and shoulder. 3 PUMPS 60.75 mg Apply 2 pumps of testosterone gel 1.62% to 1 upper arm and shoulder and then apply 1 pump of testosterone gel 1.62% to the opposite upper arm and shoulder. 4 PUMPS 81 mg Apply 2 pumps of testosterone gel 1.62% to 1 upper arm and shoulder and then apply 2 pumps of testosterone gel 1.62% to the opposite upper arm and shoulder. If you are using testosterone gel 1.62% packets:
- Tear open the packet completely at the dotted line. Squeeze from the bottom of the packet to the top.
- Squeeze all of the testosterone gel 1.62% out of the packet into the palm of your hand.
- Apply testosterone gel 1.62% to the application site. You may also apply testosterone gel 1.62% directly to the application site.
- Let the application site dry completely before putting on a t-shirt.
- Testosterone gel 1.62% is flammable until dry. Let testosterone 1.62% dry before smoking or going near an open flame.
- Avoid showering, swimming or bathing for at least 2 hours after you apply testosterone gel 1.62%. Wash your hands right away with soap and water after applying testosterone 1.62%.
Find Your Dose as
Prescribed by Your
Healthcare Provider
Application Method One 20.25 mg packet 20.25 mg Apply 1 packet of testosterone gel 1.62% to 1 upper arm and shoulder. One 40.5 mg packet 40.5 mg Apply half of the 40.5 mg packet of testosterone gel 1.62% to 1 upper arm and shoulder and then apply the remaining packet contents to the opposite upper arm and shoulder. One 40.5 mg packet and one 20.25 mg packet 60.75 mg Apply one 40.5 mg packet of testosterone gel 1.62% to 1 upper arm and shoulder and then apply one 20.25 mg packet of testosterone gel 1.62% to the opposite upper arm and shoulder. Two 40.5 mg packets 81 mg Apply one 40.5 mg packet of testosterone gel 1.62% to 1 upper arm and shoulder and then apply one 40.5 mg packet of testosterone gel 1.62% to the opposite upper arm and shoulder. How should I store testosterone gel 1.62%?
- Store testosterone gel 1.62% at 59ºF to 86ºF (15ºC to 30ºC).
- When it is time to throw away the pump or packets, safely throw away used testosterone gel 1.62% in the household trash. Be careful to prevent accidental exposure of children or pets.
- Keep testosterone gel 1.62% away from fire.
Keep testosterone gel 1.62% and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. A 8/2019
-
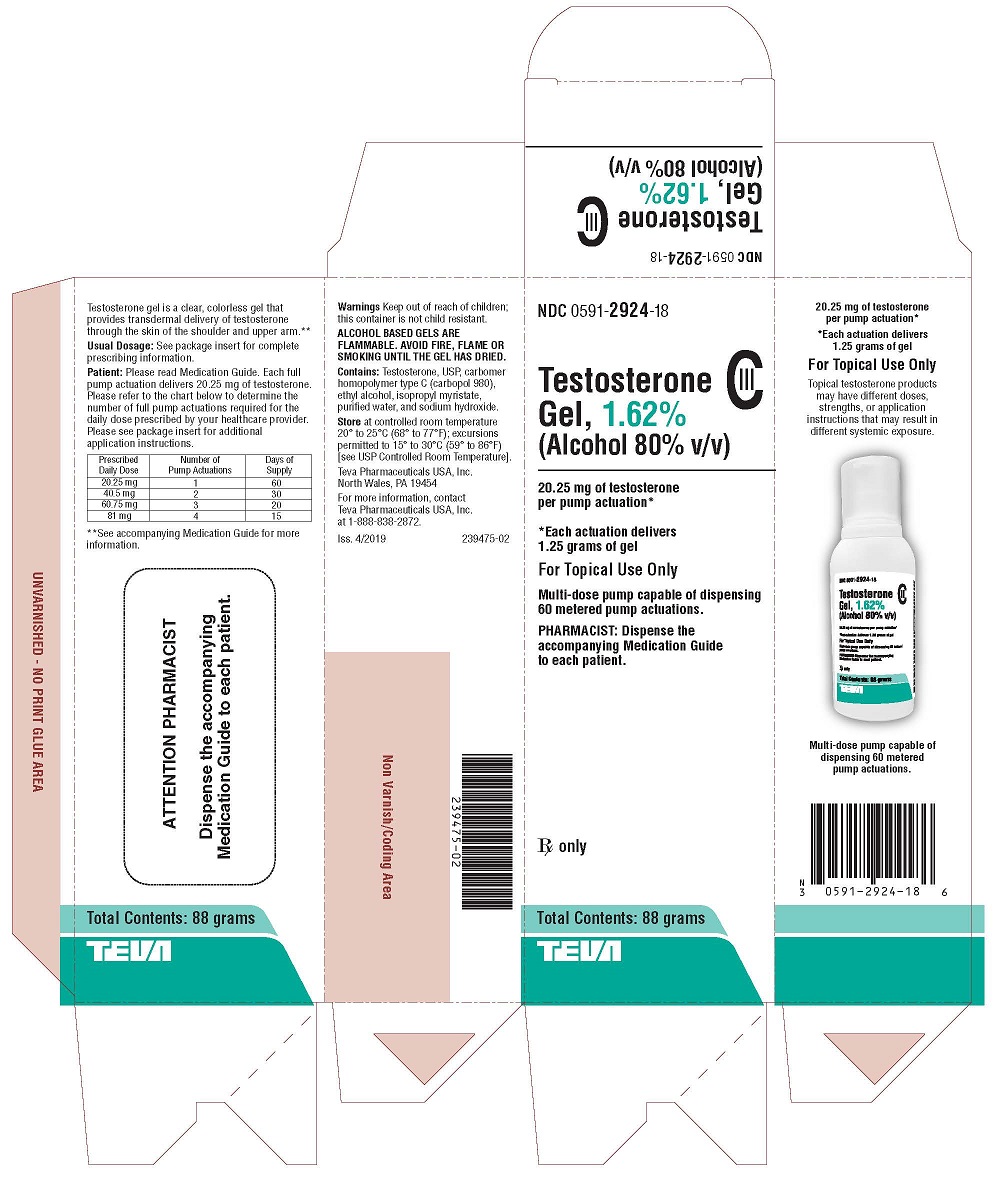
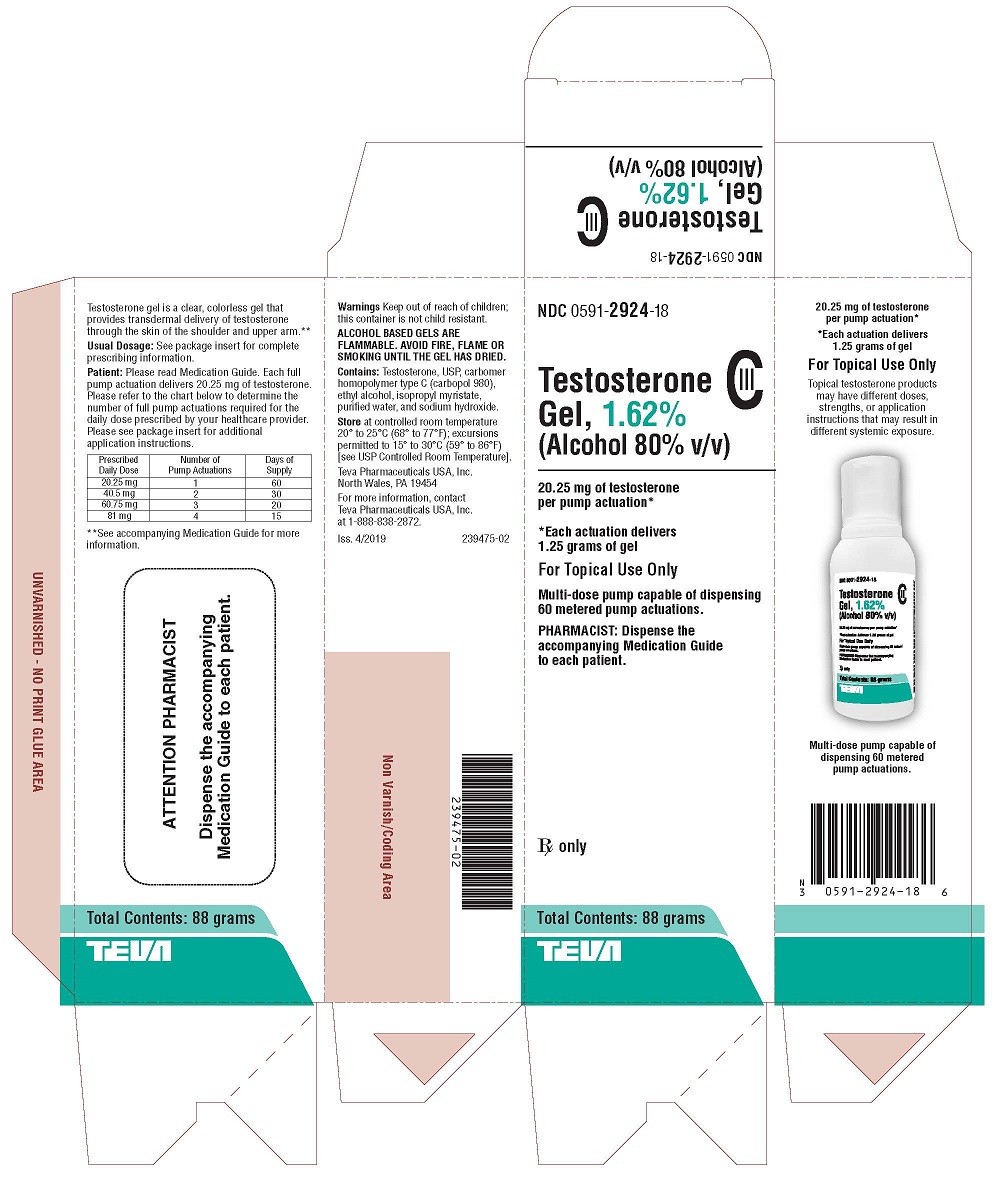
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0591-2924-18
Testosterone Gel, 1.62% CIII
(Alcohol 80% v/v)20.25 mg of testosterone per pump actuation*
*Each actuation delivers 1.25 grams of gel
FOR TOPICAL USE ONLY
Multi-dose pump capable of dispensing 60 metered pump actuations.
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
Total Contents: 88 grams
TEVA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0591-2925-30
Testosterone Gel, 1.62% CIII
(Alcohol 80% v/v)Contains 20.25 mg of testosterone, USP in 1.25 grams of gel per unit dose
Clear, colorless gel provides transdermal delivery of testosterone through the skin of the shoulders, and upper arms*
Topical testosterone products may have different doses, strengths, or application instructions that may result in different systemic exposure.PHARMACIST: Dispense the enclosed Medication Guide to each patient.
*See accompanying package insert.
For Topical Use Only
Rx only
30 Unit-dose Packets
TEVA

-
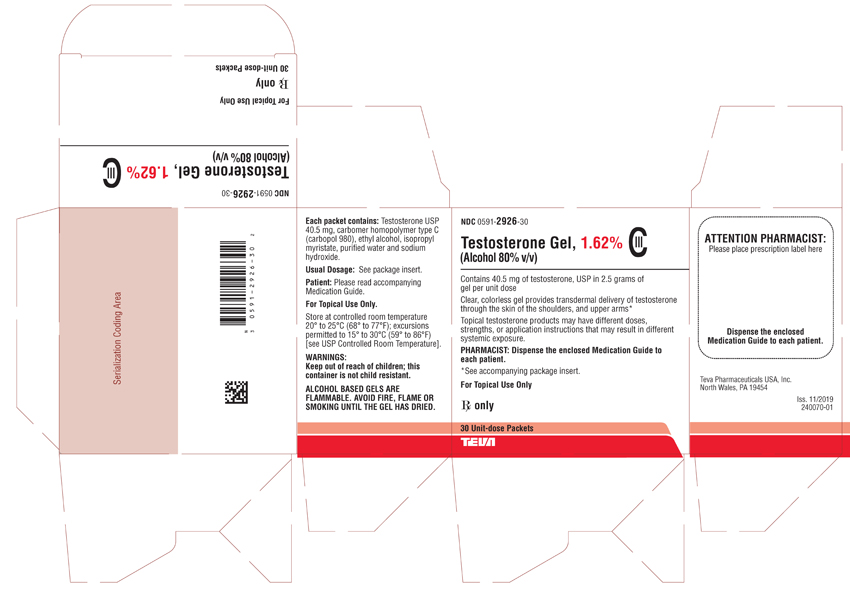
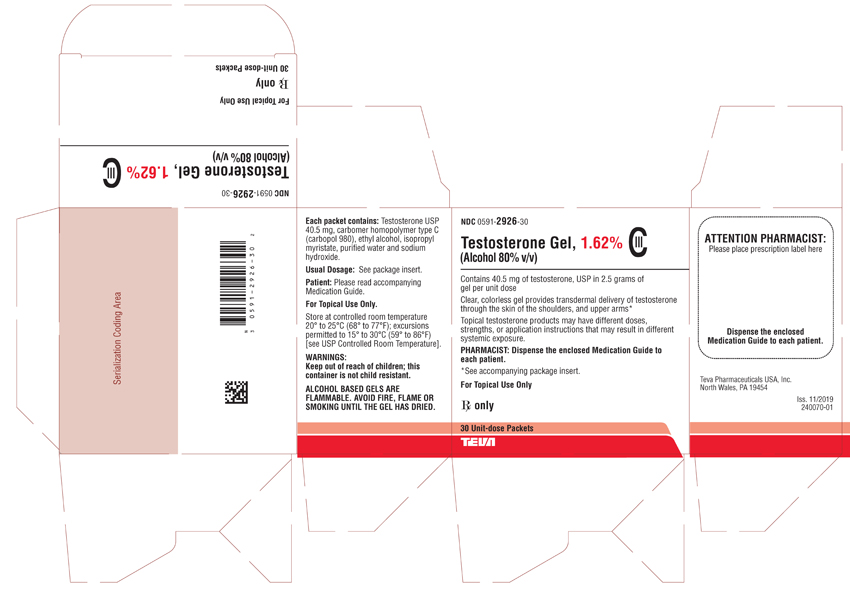
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0591-2926-30
Testosterone Gel, 1.62% CIII
(Alcohol 80% v/v)Contains 40.5 mg of testosterone, USP in 2.5 grams of gel per unit dose
Clear, colorless gel provides transdermal delivery of testosterone through the skin of the shoulders, and upper arms*
Topical testosterone products may have different doses, strengths, or application instructions that may result in different systemic exposure.PHARMACIST: Dispense the enclosed Medication Guide to each patient.
*See accompanying package insert.
For Topical Use Only
Rx only
30 Unit-dose Packets
TEVA
-
INGREDIENTS AND APPEARANCE
TESTOSTERONE
testosterone gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-2924 Route of Administration TRANSDERMAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE (UNII: 3XMK78S47O) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE 16.2 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-2924-18 1 in 1 CARTON 04/10/2019 1 88 g in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204570 04/10/2019 TESTOSTERONE
testosterone gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-2925 Route of Administration TRANSDERMAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE (UNII: 3XMK78S47O) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE 16.2 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-2925-30 30 in 1 CARTON 02/12/2021 1 NDC:0591-2925-32 1.25 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204570 02/12/2021 TESTOSTERONE
testosterone gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-2926 Route of Administration TRANSDERMAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE (UNII: 3XMK78S47O) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE 16.2 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-2926-30 30 in 1 CARTON 02/12/2021 1 NDC:0591-2926-25 2.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204570 02/12/2021 Labeler - Actavis Pharma, Inc. (119723554)