Label: QLOSI- pilocarpine hydrochloride solution
- NDC Code(s): 83661-018-10, 83661-018-20, 83661-018-30, 83661-018-60
- Packager: Orasis Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONQLOSI - These highlights do not include all the information needed to use see full prescribing information for Initial U.S. Approval - INDICATIONS AND USAGE - QLOSI is a cholinergic agonist ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEQLOSI is indicated for the treatment of presbyopia in adults.
-
2 DOSAGE AND ADMINISTRATIONInstill one drop of QLOSI in each eye. This can be repeated a second time after 2 to 3 hours for an effect up to 8 hours. QLOSI can be administered on a daily basis, or as needed, up to twice each ...
-
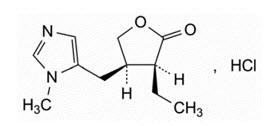
3 DOSAGE FORMS AND STRENGTHSQLOSI is a clear, colorless to slightly yellowish ophthalmic solution containing pilocarpine hydrochloride 0.4% (4 mg /mL) in a single-patient-use vial.
-
4 CONTRAINDICATIONSQLOSI is contraindicated in patients with known hypersensitivity to the active ingredient or to any of the excipients.
-
5 WARNINGS AND PRECAUTIONS5.1 Blurred Vision - Miotics, including QLOSI, may cause accommodative spasm. Advise patients to not drive or operate machinery if vision is not clear (e.g., blurred vision). In addition ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of QLOSI administration in pregnant women to inform a drug associated risk. Oral administration of pilocarpine to ...
-
10 OVERDOSAGESystemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity. Accidental ...
-
11 DESCRIPTIONQLOSI (pilocarpine hydrochloride ophthalmic solution) 0.4% is a sterile, clear, colorless to slightly yellowish and slightly viscous ophthalmic solution for topical ophthalmic use which has a pH ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pilocarpine hydrochloride is a cholinergic muscarinic agonist which activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Lifetime oral carcinogenicity studies were conducted in CD-1 mice and Sprague-Dawley rats. Pilocarpine did not ...
-
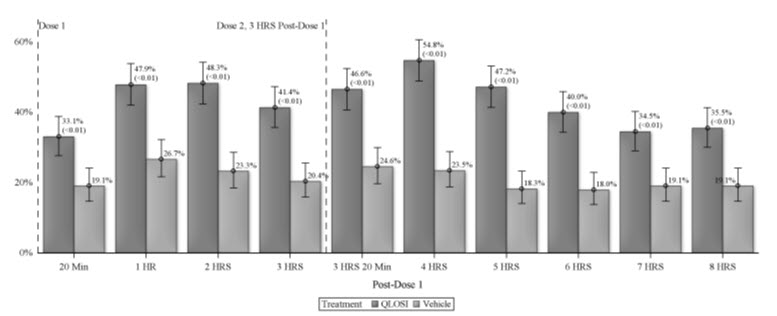
14 CLINICAL STUDIESThe efficacy of QLOSI for the treatment of presbyopia was demonstrated in two Phase 3, randomized, double-masked, vehicle-controlled studies, namely NEAR-1 (NCT04599933) and NEAR-2 (NCT04599972) ...
-
17 PATIENT COUNSELING INFORMATIONNight Driving - QLOSI may cause temporary dim or dark vision. Advise patients to exercise caution with night driving and when hazardous activities are undertaken in poor ...
-
16 HOW SUPPLIED/ STORAGE AND HANDLING16.1 How Supplied - QLOSI is supplied as a sterile, clear, colorless to slightly yellowish and slightly viscous ophthalmic solution in configurations of 5 single-patient-use vials of 0.4 mL fill ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Orasis Pharmaceuticals, Inc. Ponte Vedra, FL 32081
-
PRINCIPAL DISPLAY PANEL - 0.4 mL Vial BoxQlosi - ™ (pilocarpine HCl - ophthalmic - solution) 0.4% Sterile / Rx only - For topical application in the eye - 6 pouches containing 5 single- patient-use vials (0.4 mL each) NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information