Label: DEXTROSE- dextrose monohydrate injection, solution
- NDC Code(s): 0409-0505-15, 0409-0505-25, 0409-4902-34, 0409-4902-64, view more
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONNOTE: This solution is hypertonic - See WARNINGS and PRECAUTIONS. LifeShield® Abboject® Syringe - Abboject® Syringe - Fliptop Container - Ansyr® II Plastic Syringe - Rx only
-
DESCRIPTION50% Dextrose Injection, USP is a sterile, nonpyrogenic, hypertonic solution of dextrose in water for injection for intravenous injection as a fluid and nutrient replenisher. Each mL of fluid ...
-
CLINICAL PHARMACOLOGYWhen administered intravenously this solution restores blood glucose levels in hypoglycemia and provides a source of carbohydrate calories. Carbohydrate in the form of dextrose may aid in ...
-
INDICATIONS AND USAGE50% Dextrose Injection is indicated in the treatment of insulin hypoglycemia (hyperinsulinemia or insulin shock) to restore blood glucose levels. The solution is also indicated, after dilution ...
-
CONTRAINDICATIONSA concentrated dextrose solution should not be used when intracranial or intraspinal hemorrhage is present, nor in the presence of delirium tremens if the patient is already dehydrated. Dextrose ...
-
WARNINGS50% Dextrose Injection is hypertonic and may cause phlebitis and thrombosis at the site of injection. Significant hyperglycemia and possible hyperosmolar syndrome may result from too rapid ...
-
PRECAUTIONSDo not use unless the solution is clear and seal is intact. Discard unused portion. Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of ...
-
ADVERSE REACTIONSHyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause mental confusion and/or loss of consciousness. Reactions which may occur because of the ...
-
OVERDOSAGEIn the event of overhydration or solute overload during therapy, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
-
DOSAGE AND ADMINISTRATIONFor peripheral vein administration: Injection of the solution should be made slowly. The maximum rate at which dextrose can be infused without producing glycosuria is 0.5 g/kg of body ...
-
HOW SUPPLIED50% Dextrose Injection, USP is supplied in single-dose containers as follows: Unit of Sale and Product DescriptionStrength - (Concentration)NDC - Bundle of 10 - 50 mL LifeShield® Abboject ...
-
SPL UNCLASSIFIED SECTIONAbboject® is a trademark of Abbott Laboratories. LifeShield® is the trademark of ICU Medical, Inc. and is used under license. For Medical Information about 50% Dextrose Injection, please visit ...
-
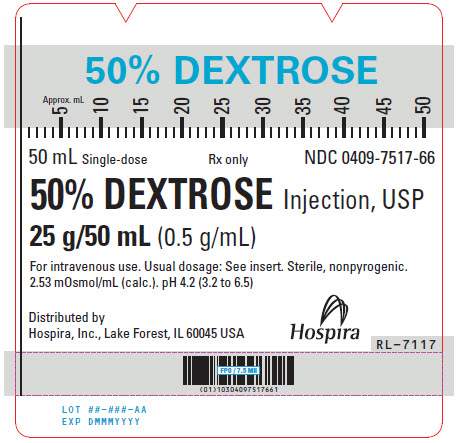
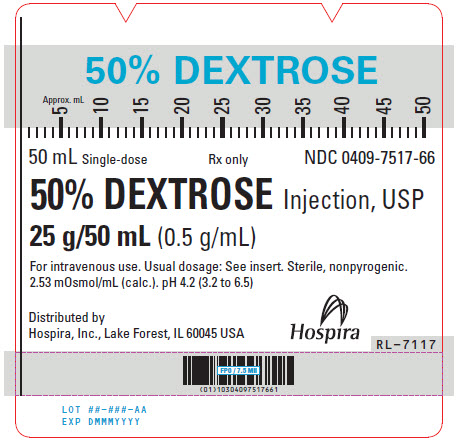
PRINCIPAL DISPLAY PANEL - 50 mL Syringe Label - 0409-7517-6650% DEXTROSE - 50 mL Single-dose - Rx only - NDC 0409-7517-66 - 50% DEXTROSE Injection, USP - 25 g/50 mL (0.5 g/mL) For intravenous use. Usual dosage: See insert. Sterile, nonpyrogenic. 2.53 mOsmol/mL ...
-
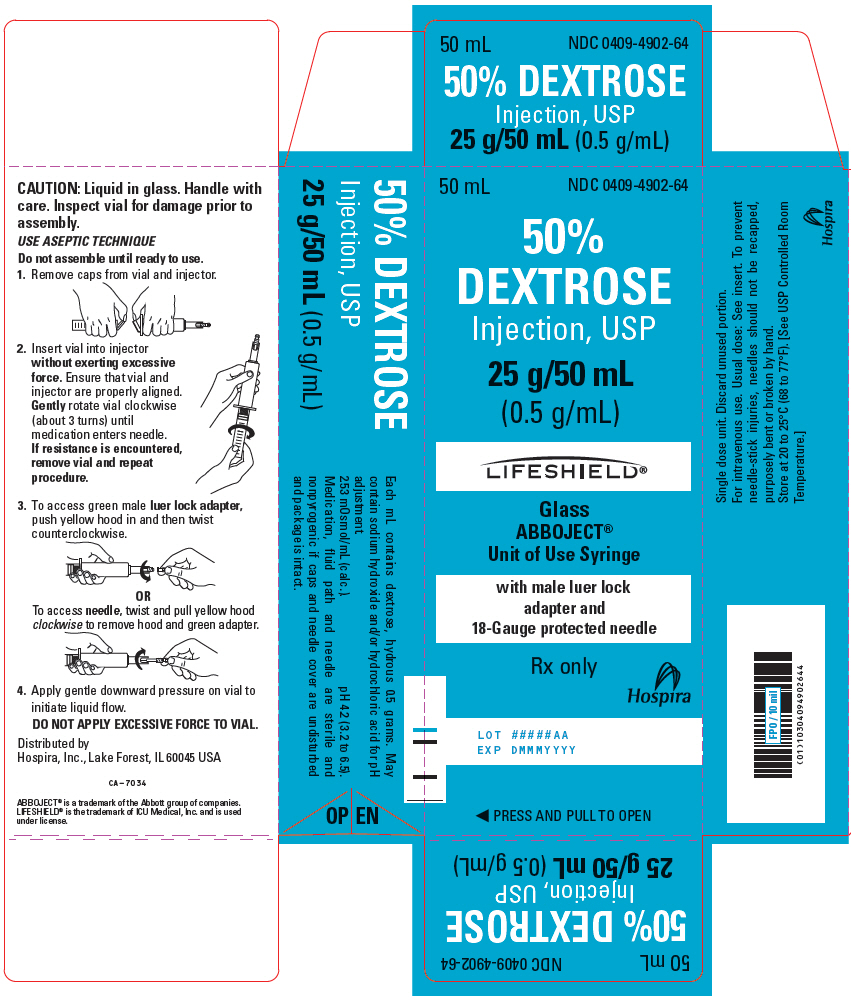
PRINCIPAL DISPLAY PANEL - 50 mL Syringe Carton - 0409-7517-6650 mL - NDC 0409-7517-66 - 50% DEXTROSE - Injection, USP - 25 g/50 mL (0.5 g/mL) Ansyr®II - Unit of Use Syringe - Rx only - LOT #####AA - EXP DMMMYYYY - Hospira - ◀ PRESS AND PULL TO OPEN ▶
-
PRINCIPAL DISPLAY PANEL - 50 mL Syringe Label - 0409-4902-6450% DEXTROSE - 50 mL Single-dose - Rx only - NDC 0409-4902-64 - 50% DEXTROSE Inj., USP - 25 g/50 mL (0.5 g/mL) For intravenous use. Usual dosage: See insert. Sterile, nonpyrogenic. 2.53 mOsmol/mL (calc. ...
-
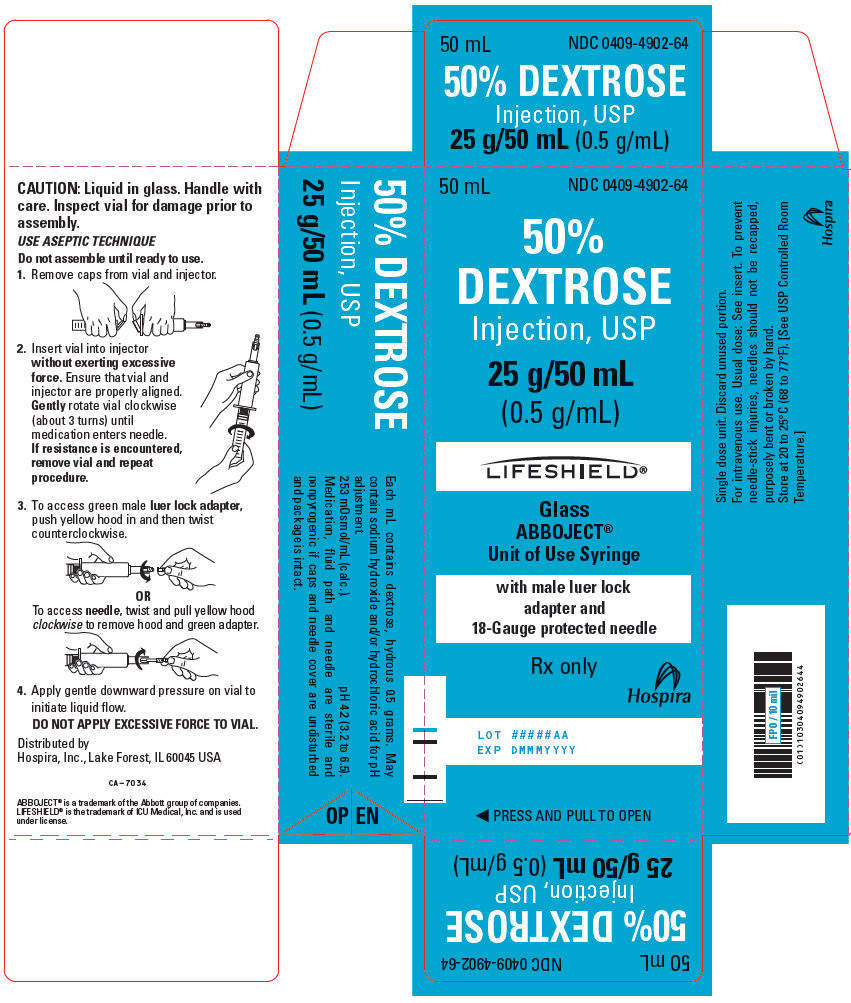
PRINCIPAL DISPLAY PANEL - 50 mL Syringe Carton - 0409-4902-6450 mL - NDC 0409-4902-64 - 50% DEXTROSE - Injection, USP - 25 g/50 mL - (0.5 g/mL) LIFESHIELD® Glass - ABBOJECT® Unit of Use Syringe - with male luer lock - adapter and - 18-Gauge protected needle - Rx ...
-
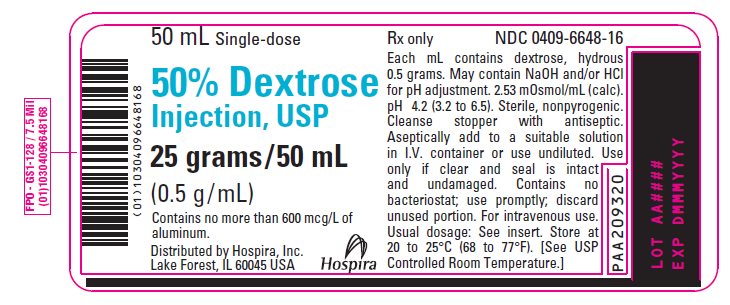
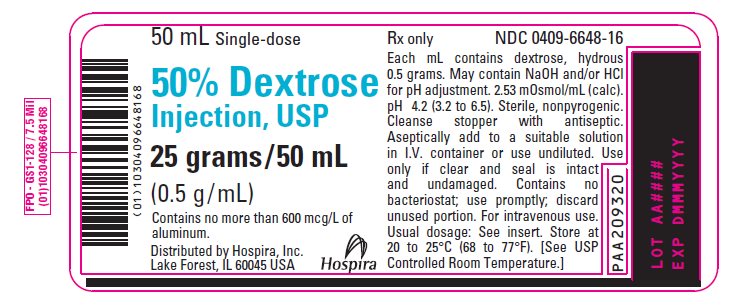
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label - 0409-6648-1650 mL Single-dose - 50% Dextrose - Injection, USP - 25 grams/50 mL - (0.5 g/mL) Contains no more than 600 mcg/L of - aluminum. Distributed by Hospira, Inc. Lake Forest, IL 60045 USA - Hospira
-
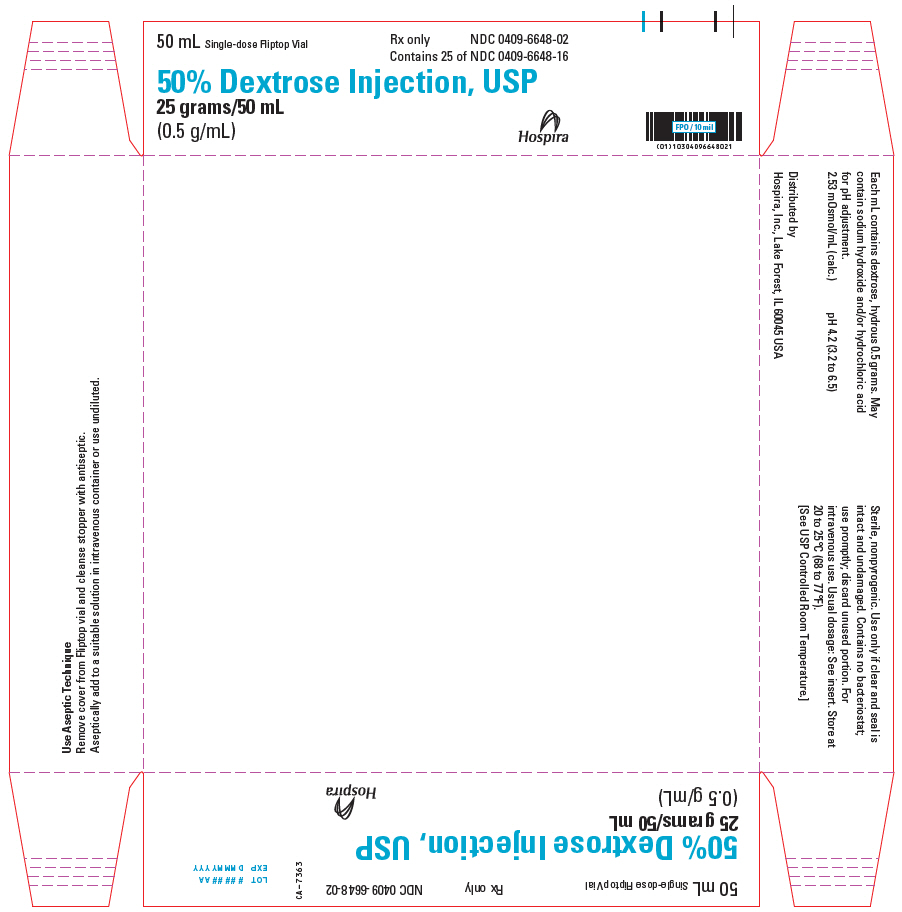
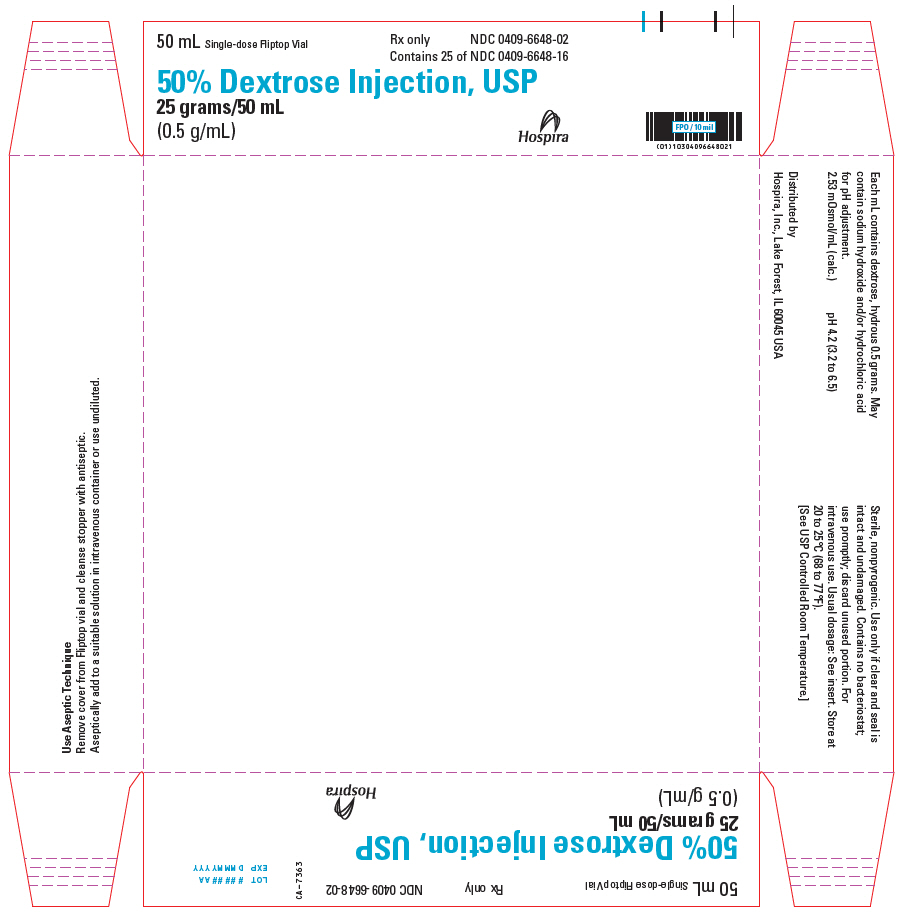
PRINCIPAL DISPLAY PANEL - 50 mL Vial Tray - 0409-6648-0250 mL Single-dose Fliptop Vial - Rx only - NDC 0409-6648-02 - Contains 25 of NDC 0409-6648-16 - 50% Dextrose Injection, USP - 25 grams/50 mL - (0.5 g/mL) Hospira
-
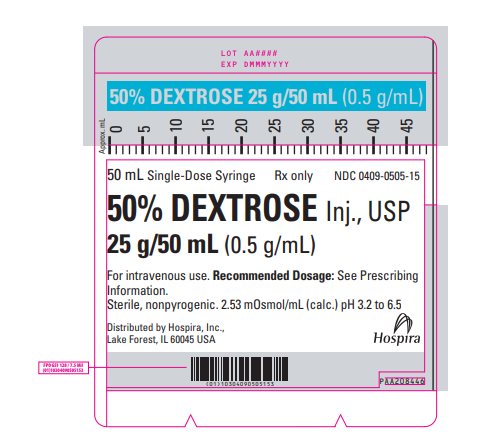
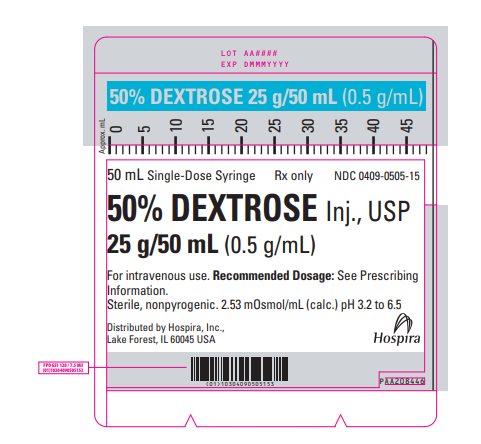
PRINCIPAL DISPLAY PANEL - 50 mL Syringe Label - 0409-0505-15 50 mL Single-Dose Syringe - Rx only - NDC 0409-0505-15 - 50% DEXTROSE Inj., USP - 25 g/50 mL (0.5 g/mL) For intravenous use. Recommended Dosage: See Prescribing - Information. Sterile, nonpyrogenic. 2.53 ...
-
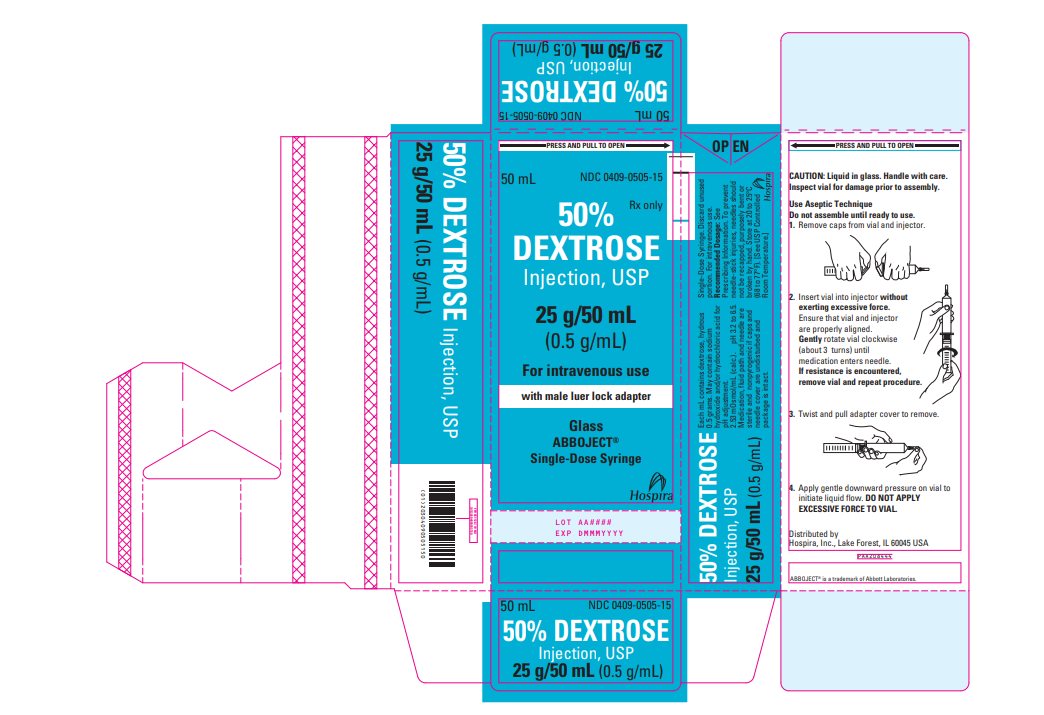
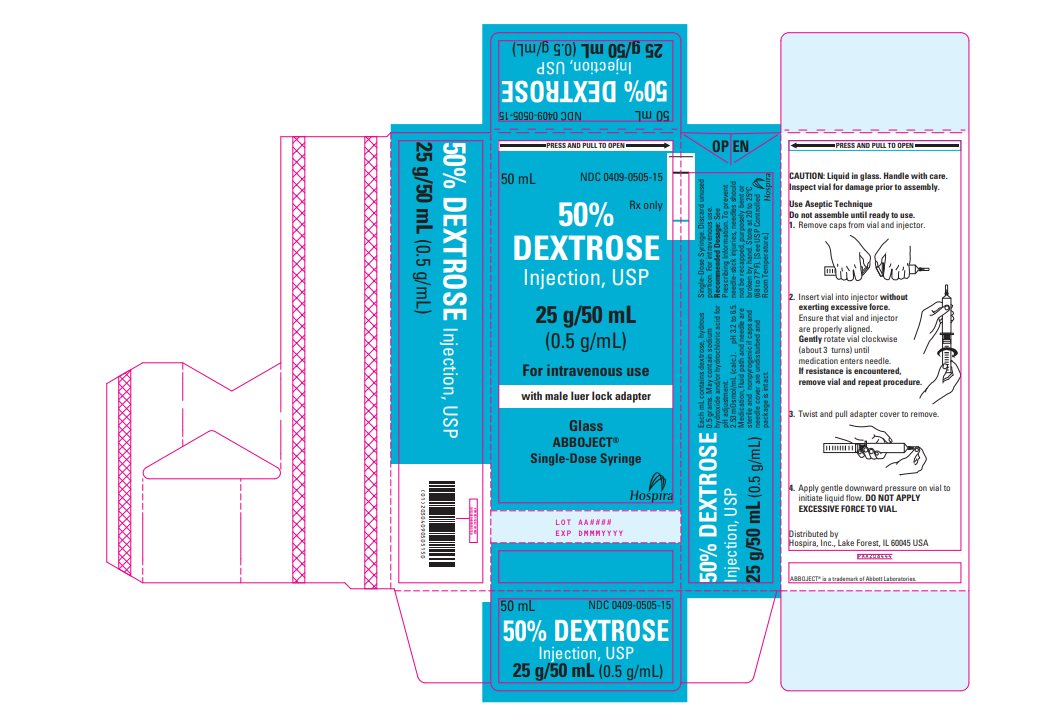
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton - 0409-0505-15 PRESS AND PULL TO OPEN - 50 mL - NDC 0409-0505-15 - Rx only - 50% DEXTROSE - Injection, USP - 25 g/50 mL - (0.5 g/mL) For intravenous use - with male luer lock adapter - Glass - Abboject® Single-Dose ...
-
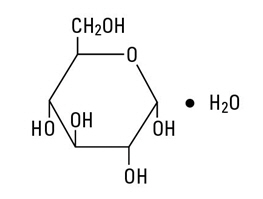
INGREDIENTS AND APPEARANCEProduct Information