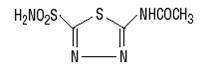
General
- Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under ...
General
Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under certain circumstances, however, very large doses have been given in conjunction with other diuretics in order to secure diuresis in complete refractory failure.
Choroidal effusion and choroidal detachment have been reported after the use of acetazolamide in the post-operative period following ophthalmic surgery. Discontinue acetazolamide if choroidal effusion and/or choroidal detachment is suspected.
Information for Patients
Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia, and agranulocytosis. Caution is advised for early detection of such reactions and the drug should be discontinued and appropriate therapy instituted.
In patients with pulmonary obstruction or emphysema where alveolar ventilation may be impaired, acetazolamide which may precipitate or aggravate acidosis should be used with caution. Gradual ascent is desirable to try to avoid acute mountain sickness. If rapid ascent is undertaken and acetazolamide is used, it should be noted that such use does not obviate the need for prompt descent if severe forms of high altitude sickness occur, i.e., high altitude pulmonary edema (HAPE) or high altitude cerebral edema.
Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported (see
WARNINGS).
Both increases and decreases in blood glucose have been described in patients treated with acetazolamide. This should be taken into consideration in patients with impaired glucose tolerance or diabetes mellitus.
Acetazolamide treatment may cause electrolyte imbalances, including hyponatremia and hypokalemia, as well as metabolic acidosis. Therefore, periodic monitoring of serum electrolytes is recommended. Particular caution is recommended in patients with conditions that are associated with, or predispose a patient to, electrolyte and acid/base imbalances, such as patients with impaired renal function (including elderly patients; see
PRECAUTIONS, Geriatric Use), patients with diabetes mellitus, and patients with impaired alveolar ventilation.
Some adverse reactions to acetazolamide, such as drowsiness, fatigue, and myopia, may impair the ability to drive and operate machinery.
Laboratory Tests
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating acetazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is recommended.
Drug Interactions
Aspirin - See
WARNINGS
Acetazolamide modifies phenytoin metabolism with increased serum levels of phenytoin. This may increase or enhance the occurrence of osteomalacia in some patients receiving chronic phenytoin therapy. Caution is advised in patients receiving chronic concomitant therapy. By decreasing the gastrointestinal absorption of primidone, acetazolamide may decrease serum concentrations of primidone and its metabolites, with a consequent possible decrease in anticonvulsant effect. Caution is advised when beginning, discontinuing, or changing the dose of acetazolamide in patients receiving primidone.
Because of possible additive effects with other carbonic anhydrase inhibitors, concomitant use is not advisable.
Acetazolamide may increase the effects of other folic acid antagonists.
Acetazolamide decreases urinary excretion of amphetamine and may enhance the magnitude and duration of their effect.
Acetazolamide reduces urinary excretion of quinidine and may enhance its effect.
Acetazolamide may prevent the urinary antiseptic effect of methenamine.
Acetazolamide increases lithium excretion and the lithium may be decreased.
Acetazolamide and sodium bicarbonate used concurrently increase the risk of renal calculus formation.
Acetazolamide may elevate cyclosporine levels.
Drug/laboratory test interactions
Sulfonamides may give false negative or decreased values for urinary phenolsulfonphthalein and phenol red elimination values for urinary protein, serum non-protein, and serum uric acid. Acetazolamide may produce an increased level of crystals in the urine.
Acetazolamide interferes with the HPLC method of assay for theophylline. Interference with the theophylline assay by acetazolamide depends on the solvent used in the extraction; acetazolamide may not interfere with other assay methods for theophylline.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of acetazolamide have not been conducted. In a bacterial mutagenicity assay, acetazolamide was not mutagenic when evaluated with and without metabolic activation.
The drug had no effect on fertility when administered in the diet to male and female rats at a daily intake of up to 4 times the recommended human dose of 1000 mg in a 50 kg individual.
Pregnancy: Teratogenic effects
Acetazolamide, administered orally or parenterally, has been shown to be teratogenic (defects of the limbs) in mice, rats, hamsters, and rabbits. There are no adequate and well-controlled studies in pregnant women. Acetazolamide should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from acetazolamide, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother. Acetazolamide should only be used by nursing women if the potential benefit justifies the potential risk to the child.
Pediatric Use
The safety and effectiveness of acetazolamide extended-release capsules in pediatric patients below the age of 12 years have not been established. Growth retardation has been reported in children receiving long-term therapy, believed secondary to chronic acidosis.
Geriatric Use
Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
Close