Label: PHENOBARBITAL tablet
- NDC Code(s): 70518-4129-0
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 69367-626
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - WARNING: MAY BE HABIT-FORMING
-
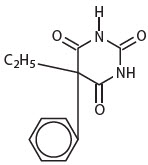
DESCRIPTIONThe barbiturates are nonselective central nervous system (CNS) depressants that are primarily used as sedative-hypnotics. In subhypnotic doses, they are also used as anticonvulsants. The ...
-
CLINICAL PHARMACOLOGYBarbiturates are capable of producing all levels of CNS mood alteration, from excitation to mild sedation, hypnosis, and deep coma. Overdosage can produce death. In high enough therapeutic doses ...
-
INDICATIONS AND USAGEA. Sedative - B. Anticonvulsant – For the treatment of generalized and partial seizures.
-
CONTRAINDICATIONSPhenobarbital is contraindicated in patients who are hypersensitive to barbiturates, in patients with a history of manifest or latent porphyria, and in patients with marked impairment of liver ...
-
WARNINGS1. Habit Forming - Phenobarbital may be habit forming. Tolerance and psychological and physical dependence may occur with continued use (see - DRUG ABUSE AND DEPENDENCEand ...
-
PRECAUTIONSGeneral - Barbiturates may be habit forming. Tolerance and psychological and physical dependence may occur with continued use (see - DRUG ABUSE AND DEPENDENCE). Barbiturates should be ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported: CNS Depression– Residual sedation or "hangover", drowsiness, lethargy, and vertigo. Emotional disturbances and phobias may be accentuated. In ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - Phenobarbital is a Schedule IV drug. Dependence - Barbiturates may be habit forming. Tolerance, psychological dependence, and physical dependence may occur, especially ...
-
OVERDOSAGESigns and Symptoms - The onset of symptoms following a toxic oral exposure to phenobarbital may not occur until several hours following ingestion. The toxic dose of barbiturates varies ...
-
DOSAGE AND ADMINISTRATIONThe dose of phenobarbital must be individualized with full knowledge of its particular characteristics. Factors of consideration are the patient's age, weight, and condition. Sedation - For ...
-
HOW SUPPLIEDPhenobarbital Tablets, USP 32.4 mg (1/2 grain) are white, round, biconvex, scored tablets, debossed "626" below the score on one side and "ECI" on the reverse side, supplied in - NDC ...
-
SPL UNCLASSIFIED SECTIONRepackaged and Distributed By: Remedy Repack, Inc. 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
PRINCIPAL DISPLAY PANELDRUG: Phenobarbital - GENERIC: Phenobarbital - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-4129-0 - COLOR: white - SHAPE: ROUND - SCORE: Two even pieces - SIZE: 6 mm - IMPRINT: 626;ECI - PACKAGING: 30 in 1 ...
-
INGREDIENTS AND APPEARANCEProduct Information