Label: DONNATAL- phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide elixir
-
NDC Code(s):
59212-422-04,
59212-422-10,
59212-422-16,
59212-423-04, view more59212-423-10, 59212-423-16
- Packager: Advanz Pharma (US) Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Donnatal® Elixir - Grape
Each 5 mL (teaspoonful) of elixir (alcohol not more than 23.8%) contains:
Phenobarbital, USP.............................. 16.2 mg
Hyoscyamine Sulfate, USP............... 0.1037 mg
Atropine Sulfate, USP...................... 0.0194 mg
Scopolamine Hydrobromide, USP.... 0.0065 mg -
DESCRIPTION
Donnatal® Elixir - Mint
Each 5 mL (teaspoonful) of elixir (alcohol not more than 23.8%) contains:
Phenobarbital, USP.............................. 16.2 mg
Hyoscyamine Sulfate, USP............... 0.1037 mg
Atropine Sulfate, USP...................... 0.0194 mg
Scopolamine Hydrobromide, USP.... 0.0065 mg - CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Based on a review of this drug by the National Academy of Sciences-National Research Council and/or other information, FDA has classified the indications as follows: "Possibly" effective: For use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis.
May also be useful as adjunctive therapy in the treatment of duodenal ulcer.
Final classification of the less-than-effective indications requires further investigation.
IT HAS NOT BEEN SHOWN CONCLUSIVELY WHETHER ANTICHOLINERGIC/ANTISPASMODIC DRUGS AID IN THE HEALING OF A DUODENAL ULCER, DECREASE THE RATE OF RECURRENCES OR PREVENT COMPLICATIONS.
-
CONTRAINDICATIONS
- glaucoma;
- obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy);
- obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis, etc.);
- paralytic ileus, intestinal atony of the elderly or debilitated patient;
- unstable cardiovascular status in acute hemorrhage;
- severe ulcerative colitis especially if complicated by toxic megacolon;
- myasthenia gravis;
- hiatal hernia associated with reflux esophagitis;
- in patients with known hypersensitivity to any of the ingredients.
Phenobarbital is contraindicated in acute intermittent porphyria and in those patients in whom phenobarbital produces restlessness and/or excitement.
-
WARNINGS
Donnatal® Elixir can cause fetal harm when administered to a pregnant woman. Animal reproduction studies have not been conducted with Donnatal® Elixir. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
In the presence of a high environmental temperature, heat prostration can occur with belladonna alkaloids (fever and heatstroke due to decreased sweating).
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
Donnatal® Elixir may produce drowsiness or blurred vision. The patient should be warned, should these occur, not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery, and not to perform hazardous work.
Phenobarbital may decrease the effect of anticoagulants, and necessitate larger doses of the anticoagulant for optimal effect. When the phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased.
Phenobarbital may be habit forming and should not be administered to individuals known to be addiction prone or to those with a history of physical and/or psychological dependence upon drugs.
Since barbiturates are metabolized in the liver, they should be used with caution and initial doses should be small in patients with hepatic dysfunction.
-
PRECAUTIONS
General
Use with caution in patients with:
- autonomic neuropathy
- hepatic or renal disease
- hyperthyroidism
- coronary heart disease
- congestive heart failure
- cardiac arrhythmias
- tachycardia
- hypertension
Belladonna alkaloids may produce a delay in gastric emptying (antral stasis) which would complicate the management of gastric ulcer.
Do not rely on the use of the drug in the presence of complication of biliary tract disease.
Theoretically, with overdosage, a curare-like action may occur.
Donnatal® Elixir – Mint contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Information for Patients
Donnatal® Elixir may produce drowsiness or blurred vision. The patient should be warned, should these occur, not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery, and not to perform hazardous work.
Drug Interactions
Phenobarbital may decrease the effect of anticoagulants, and necessitate larger doses of the anticoagulant for optimal effect. When the phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy
Animal reproduction studies have not been conducted with Donnatal® Elixir. There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks (see WARNINGS).
-
ADVERSE REACTIONS
Adverse reactions may include xerostomia; urinary hesitancy and retention; blurred vision; tachycardia; palpitation; mydriasis; cycloplegia; increased ocular tension; loss of taste sense; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; musculoskeletal pain; severe allergic reaction or drug idiosyncrasies, including anaphylaxis, urticaria, and other dermal manifestations; and decreased sweating.
Acquired hypersensitivity to barbiturates consists chiefly in allergic reactions that occur especially in persons who tend to have asthma, urticaria, angioedema, and similar conditions. Hypersensitivity reactions in this category include localized swelling, particularly of the eyelids, cheeks, or lips, and erythematous dermatitis. Rarely, exfoliative dermatitis (e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis) may be caused by phenobarbital and can prove fatal. The skin eruption may be associated with fever, delirium, and marked degenerative changes in the liver and other parenchymatous organs. In a few cases, megaloblastic anemia has been associated with the chronic use of phenobarbital.
Phenobarbital may produce excitement in some patients, rather than a sedative effect.
To report SUSPECTED ADVERSE REACTIONS, contact Advanz Pharma (US) Corp. at 1-877-370-1142 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DRUG ABUSE AND DEPENDENCE
Abuse
Phenobarbital may be habit forming and should not be administered to individuals known to be addiction prone or to those with a history of physical and/or psychological dependence upon drugs (see WARNINGS).
-
OVERDOSAGE
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot and dry skin, dizziness, dryness of the mouth, difficulty in swallowing, and CNS stimulation. Treatment should consist of gastric lavage, emetics, and activated charcoal. If indicated, parenteral cholinergic agents such as physostigmine or bethanechol chloride should be used.
-
DOSAGE AND ADMINISTRATION
The dosage of Donnatal® Elixir should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse effects.
Donnatal® Elixir. Adults: One or two teaspoonfuls of elixir three or four times a day according to conditions and severity of symptoms.
Pediatric patients: may be dosed every 4 to 6 hours. Use a pediatric dosing device or oral syringe to measure the dose.
Starting Dosage Body weight Every 4 hours Every 6 hours 10 lb. (4.5 kg) 0.5 mL 0.75 mL 20 lb. (9.1 kg) 1 mL 1.5 mL 30 lb. (13.6 kg) 1.5 mL 2 mL 50 lb. (22.7 kg) 2.5 mL 3.75 mL 75 lb. (34 kg) 3.75 mL 5 mL 100 lb. (45.4 kg) 5 mL 7.5 mL -
HOW SUPPLIED
Donnatal® Elixir - Grape is a purple colored, grape flavored liquid.
- 4 fl oz (118 mL) bottles- NDC 59212-423-04.
- 1 Pint (473 mL) bottles- NDC 59212-423-16.
Donnatal® Elixir - Mint is a green colored, mint flavored liquid.
- 4 fl oz (118 mL) bottles- NDC 59212-422-04.
- 1 Pint (473 mL) bottles- NDC 59212-422-16.
Avoid Freezing
Store Donnatal® Elixir at 20º- 25ºC (68º - 77ºF) [see USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
DEA EXEMPT PRODUCT
Manufactured for:
Advanz Pharma (US) Corp.
Bannockburn, IL 60015
DONNATAL® is a registered trademark of Mercury Pharma Group Limited.
Distributed by Advanz Pharma (US) Corp. under license.
Revised:11/23
-
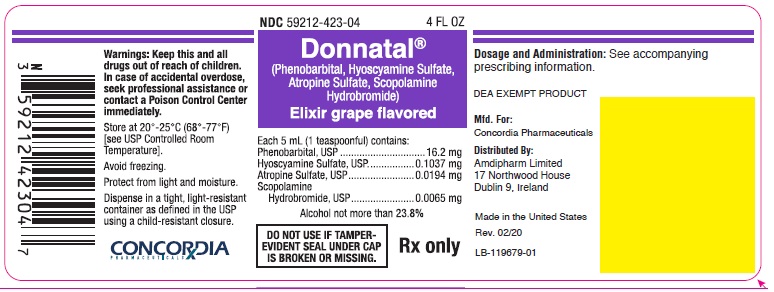
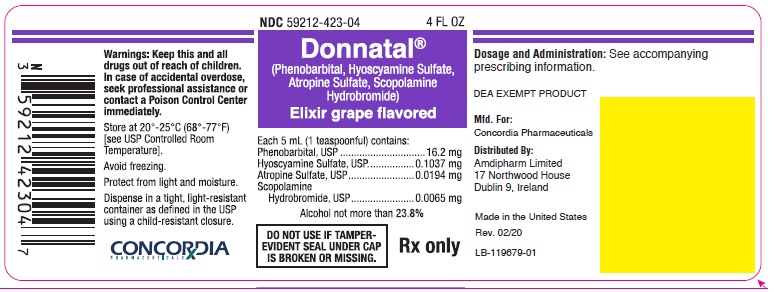
Principal Display Panel - Donnatal Elixir - Grape, 4 oz
NDC 59212-423-04 4 FL OZ
Donnatal®
(Phenobarbital, Hyoscyamine Sulfate, Atropine Sulfate, Scopolamine Hydrobromide)Elixir grape flavored
Each 5 mL (1 teaspoonful) contains:
Phenobarbital, USP.............................. 16.2 mg
Hyoscyamine Sulfate, USP............... 0.1037 mg
Atropine Sulfate, USP...................... 0.0194 mg
Scopolamine Hydrobromide, USP.... 0.0065 mgAlcohol not more than 23.8%
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER CAP IS BROKEN OR MISSING.
Rx only
-
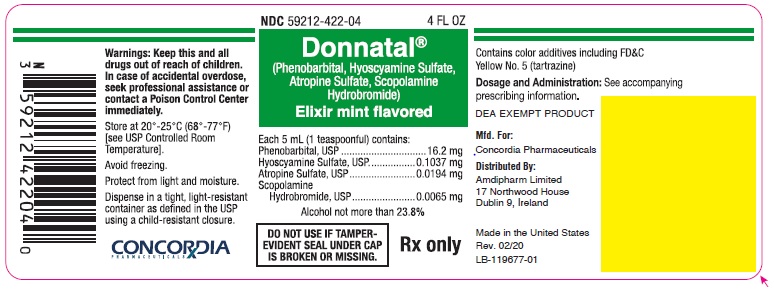
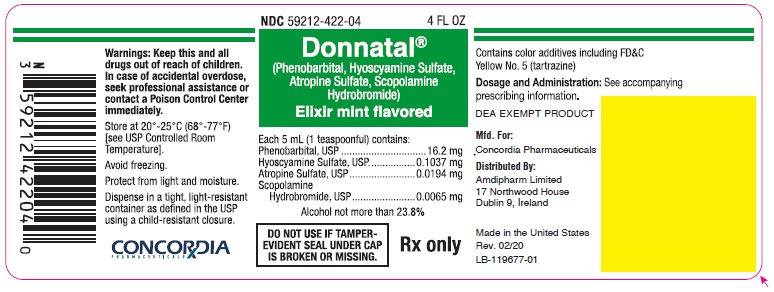
Principal Display Panel - Donnatal Elixir - Mint, 4 oz
NDC 59212-422-04 4 FL OZ
Donnatal®
(Phenobarbital, Hyoscyamine Sulfate, Atropine Sulfate, Scopolamine Hydrobromide)Elixir mint flavored
Each 5 mL (1 teaspoonful) contains:
Phenobarbital, USP.............................. 16.2 mg
Hyoscyamine Sulfate, USP............... 0.1037 mg
Atropine Sulfate, USP...................... 0.0194 mg
Scopolamine Hydrobromide, USP.... 0.0065 mgAlcohol not more than 23.8%
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER CAP IS BROKEN OR MISSING.
Rx only
-
INGREDIENTS AND APPEARANCE
DONNATAL
phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide elixirProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59212-423 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenobarbital (UNII: YQE403BP4D) (Phenobarbital - UNII:YQE403BP4D) Phenobarbital 16.2 mg in 5 mL Hyoscyamine sulfate (UNII: F2R8V82B84) (Hyoscyamine - UNII:PX44XO846X) Hyoscyamine sulfate 0.1037 mg in 5 mL Atropine sulfate (UNII: 03J5ZE7KA5) (Atropine - UNII:7C0697DR9I) Atropine sulfate 0.0194 mg in 5 mL Scopolamine hydrobromide (UNII: 451IFR0GXB) (Scopolamine - UNII:DL48G20X8X) Scopolamine hydrobromide 0.0065 mg in 5 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) alcohol (UNII: 3K9958V90M) water (UNII: 059QF0KO0R) sorbitol (UNII: 506T60A25R) sucrose (UNII: C151H8M554) saccharin sodium (UNII: SB8ZUX40TY) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Red No. 3 (UNII: PN2ZH5LOQY) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE (Artificial and Natural Grape) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59212-423-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 2 NDC:59212-423-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 3 NDC:59212-423-10 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/30/1980 DONNATAL
phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide elixirProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59212-422 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenobarbital (UNII: YQE403BP4D) (Phenobarbital - UNII:YQE403BP4D) Phenobarbital 16.2 mg in 5 mL Hyoscyamine sulfate (UNII: F2R8V82B84) (Hyoscyamine - UNII:PX44XO846X) Hyoscyamine sulfate 0.1037 mg in 5 mL Atropine sulfate (UNII: 03J5ZE7KA5) (Atropine - UNII:7C0697DR9I) Atropine sulfate 0.0194 mg in 5 mL Scopolamine hydrobromide (UNII: 451IFR0GXB) (Scopolamine - UNII:DL48G20X8X) Scopolamine hydrobromide 0.0065 mg in 5 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) alcohol (UNII: 3K9958V90M) water (UNII: 059QF0KO0R) sorbitol (UNII: 506T60A25R) sucrose (UNII: C151H8M554) saccharin sodium (UNII: SB8ZUX40TY) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN Score Shape Size Flavor MINT (Natural mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59212-422-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 2 NDC:59212-422-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 3 NDC:59212-422-10 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/30/1980 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/30/1980 Labeler - Advanz Pharma (US) Corp. (118997081)