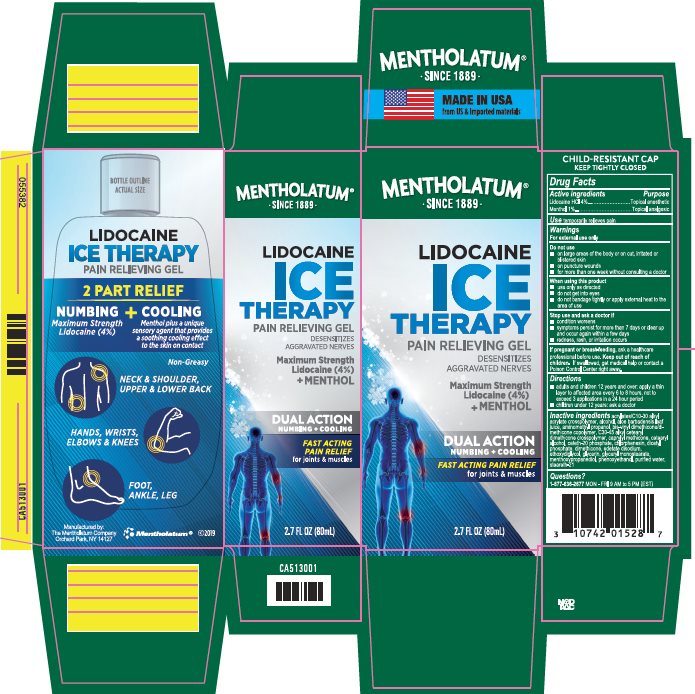
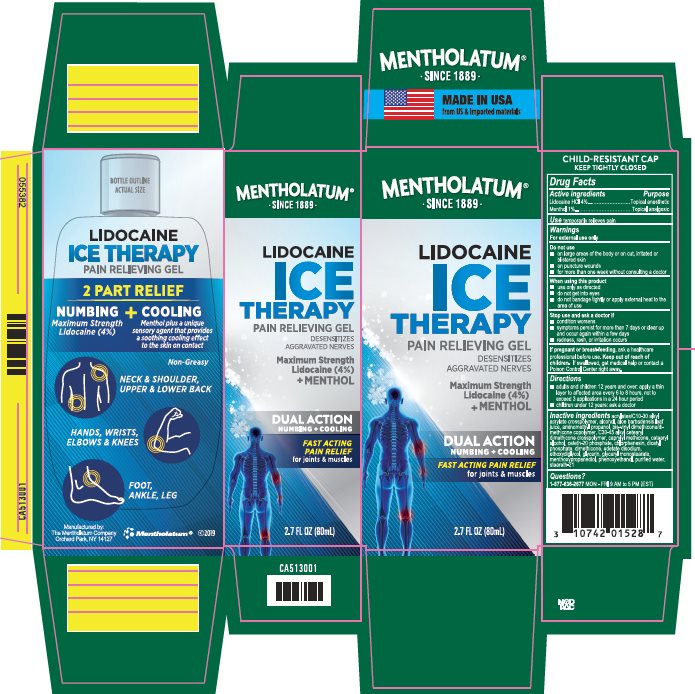
Label: MENTHOLATUM LIDOCAINE ICE- lidocaine hcl, menthol gel
- NDC Code(s): 10742-1389-1
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, alcohol, aloe barbadensis leaf juice, aminomethyl propanol, bis-vinyl dimethicone/dimecthicone copolymer, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, chlorphenesin, dicetyl phosphate, dimethicone, edetate disodium, ethoxydiglycol, glycerin, glyceryl monostearate, methoxypropanediol, phenoxyethanol, purified water, steareth-21
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MENTHOLATUM LIDOCAINE ICE
lidocaine hcl, menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-1389 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 40 mg in 1 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALCOHOL (UNII: 3K9958V90M) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETETH-20 PHOSPHATE (UNII: 921FTA1500) CHLORPHENESIN (UNII: I670DAL4SZ) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN METHYL ETHER (UNII: 42ESM1DR47) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) STEARETH-21 (UNII: 53J3F32P58) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-1389-1 1 in 1 CARTON 08/01/2018 1 80 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 08/01/2018 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-1389)