Label: DIMETHYL FUMARATE capsule, delayed release
DIMETHYL FUMARATE kit
- NDC Code(s): 69238-1318-4, 69238-1319-6, 69238-1626-3
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DIMETHYL FUMARATE DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for DIMETHYL FUMARATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDimethyl fumarate delayed-release capsules are indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and ...
-
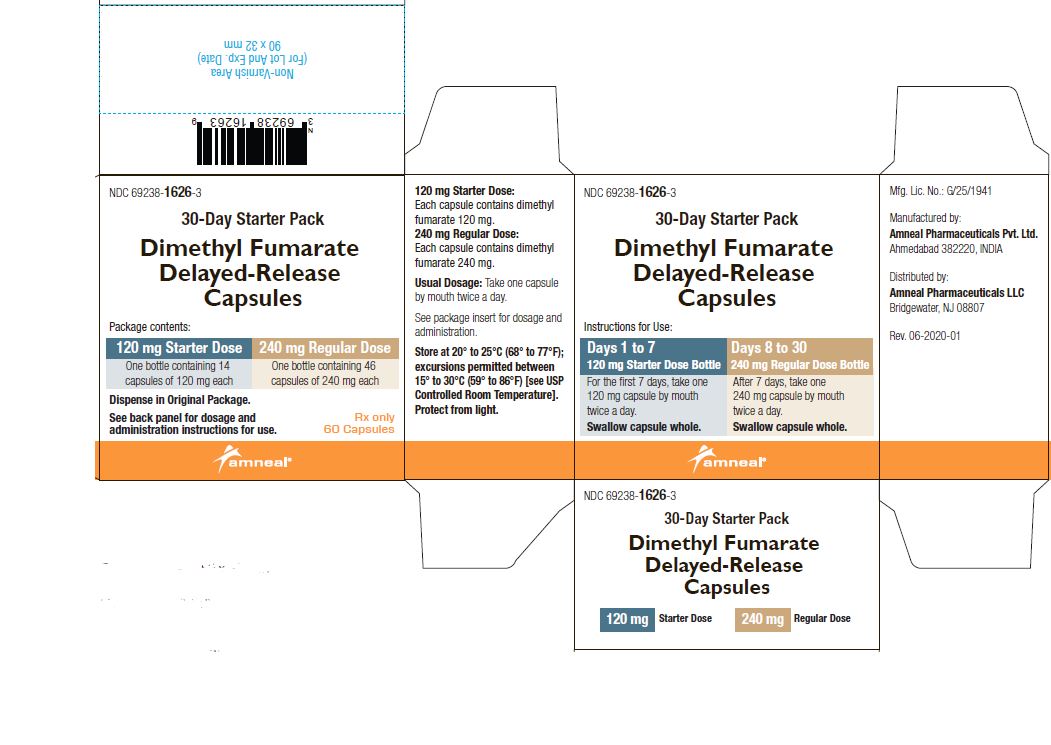
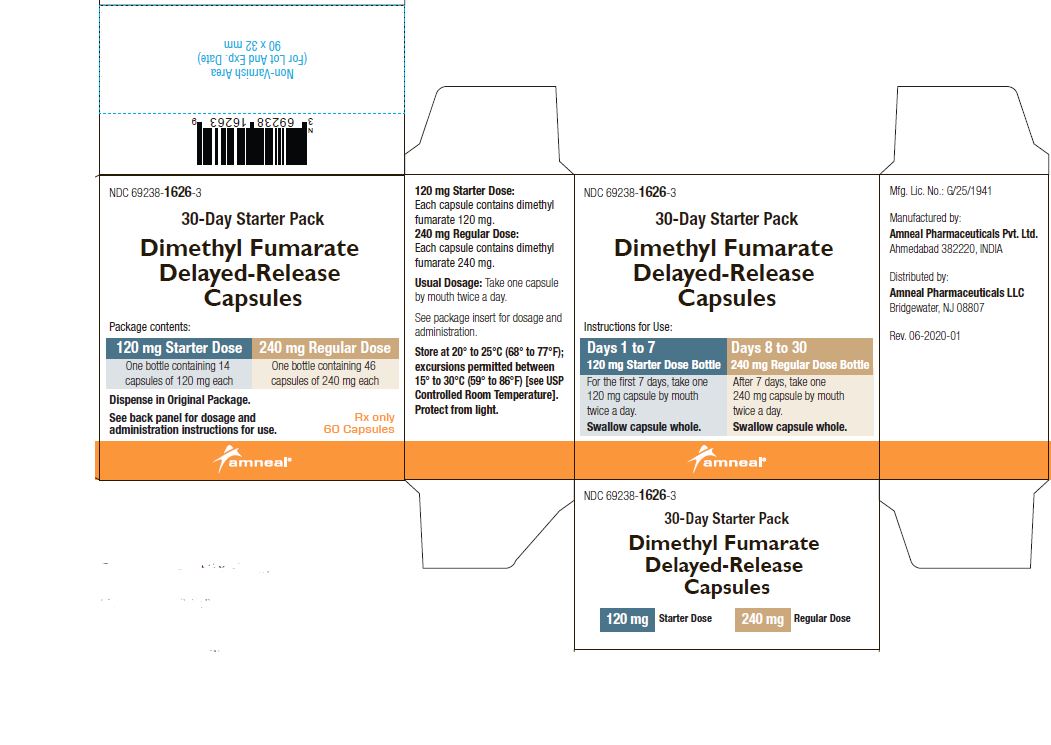
2 DOSAGE AND ADMINISTRATION2.1 Dosing - Information - The starting dose for dimethyl fumarate delayed-release capsules are 120 mg twice a day orally. After 7 days, the dose should be increased to the maintenance dose of 240 ...
-
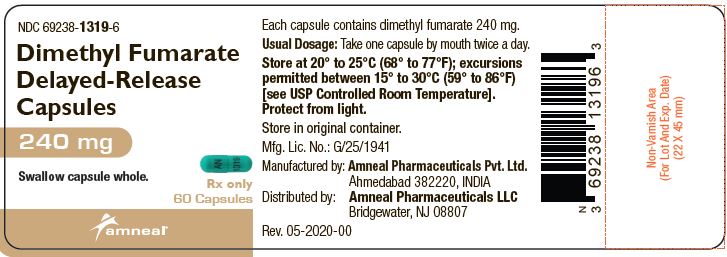
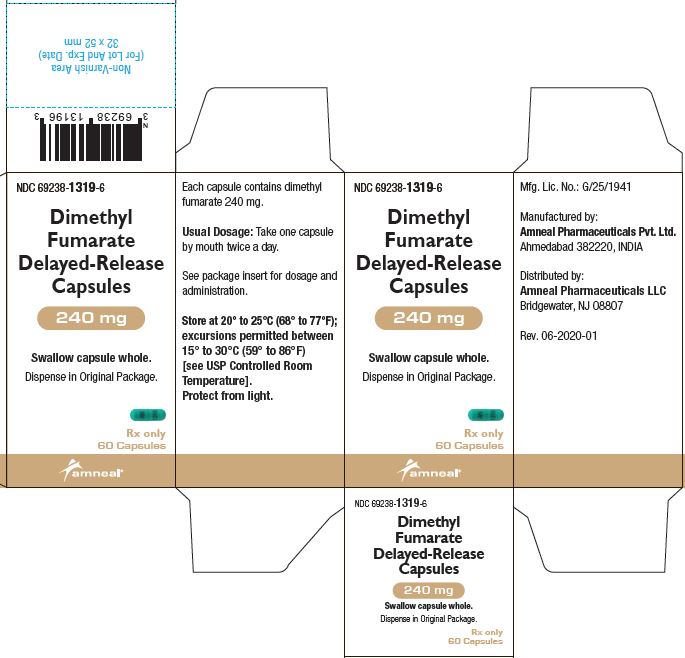
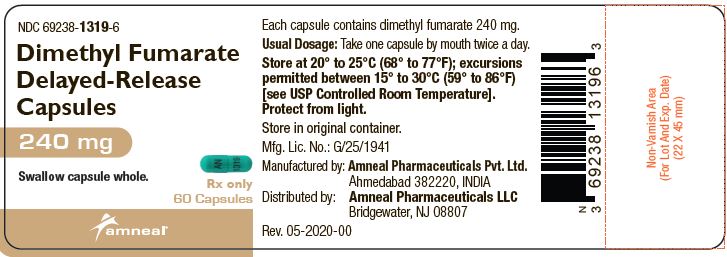
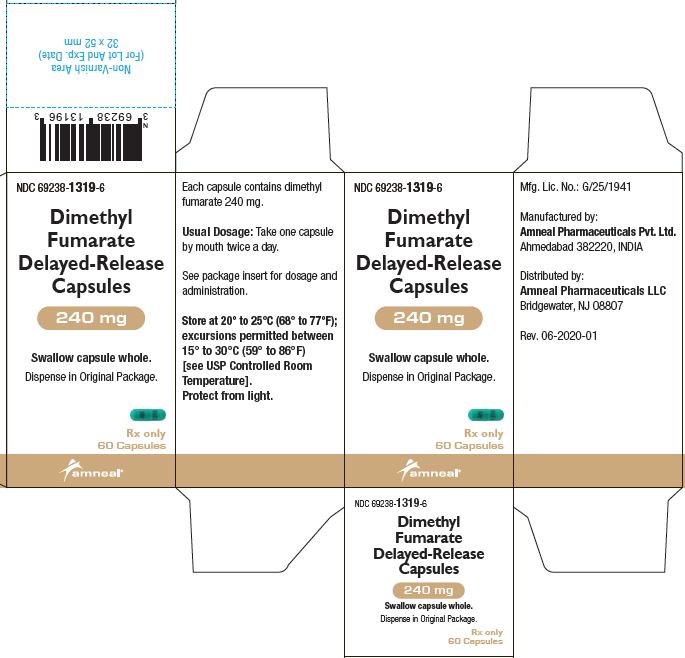
3 DOSAGE FORMS AND STRENGTHSDimethyl fumarate delayed-release capsules, USP are available as hard gelatin delayed-release capsules containing 120 mg or 240 mg of dimethyl fumarate, USP. Dimethyl Fumarate Delayed-Release ...
-
4 CONTRAINDICATIONSDimethyl fumarate delayed-release capsule are contraindicated in patients with known hypersensitivity to dimethyl fumarate or to any of the excipients of dimethyl fumarate delayed-release capsule ...
-
5 WARNINGS AND PRECAUTIONS5.1 - Anaphylaxis and Angioedema - Dimethyl fumarate can cause anaphylaxis and angioedema after the first dose or at any time during treatment. Signs and symptoms have included difficulty ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described elsewhere in labeling: Anaphylaxis and Angioedema [see Warnings and Precautions (5.1)]. Progressive multifocal leukoencephalopathy [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from the dimethyl fumarate Pregnancy Registry, observational studies, and pharmacovigilance with dimethyl fumarate use in pregnant women have not ...
-
10 OVERDOSECases of overdose with dimethyl fumarate have been reported. The symptoms described in these cases were consistent with the known adverse event profile of dimethyl fumarate. There are no known ...
-
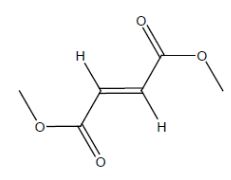
11 DESCRIPTIONDimethyl fumarate delayed-released capsules, USP contain dimethyl fumarate, USP which is also known by its chemical name, dimethyl (E) butenedioate, (C6H8O4). It has the following ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism by which dimethyl fumarate (DMF) exerts its therapeutic effect in multiple sclerosis is unknown. DMF and the metabolite, monomethyl fumarate (MMF), have ...
-
13 NONCLINICAL TOXICOLOGY13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies of dimethyl fumarate (DMF) were conducted in mice and rats. In mice, oral administration of ...
-
14 CLINICAL STUDIESThe efficacy and safety of dimethyl fumarate were demonstrated in two studies (Studies 1 and 2) that evaluated dimethyl fumarate taken either twice or three times a day in patients with ...
-
16 HOW SUPPLIED/STORAGE
AND HANDLINGDimethyl fumarate delayed-release capsules, USP are available as hard gelatin delayed-release capsules in two strengths containing either 120 mg or 240 mg of dimethyl fumarate, USP. Dimethyl ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Dosage - Inform patients that they will be provided two strengths of dimethyl fumarate delayed-release capsules ...
-
PATIENT PACKAGE INSERTPatient Information - Dimethyl Fumarate (dye meth' il fue' ma rate) Delayed-Release Capsules, USP - What are dimethyl fumarate delayed-release capsules? Dimethyl fumarate ...
-
PRINCIPAL DISPLAY PANELNDC 69238-1318-4 - Dimethyl Fumarate Delayed-Release Capsules, 120 mg - Rx only - 14 Capsules - Amneal Pharmaceuticals LLC - NDC 69238-1319-6 - Dimethyl Fumarate Delayed-Release Capsules ...
-
INGREDIENTS AND APPEARANCEProduct Information