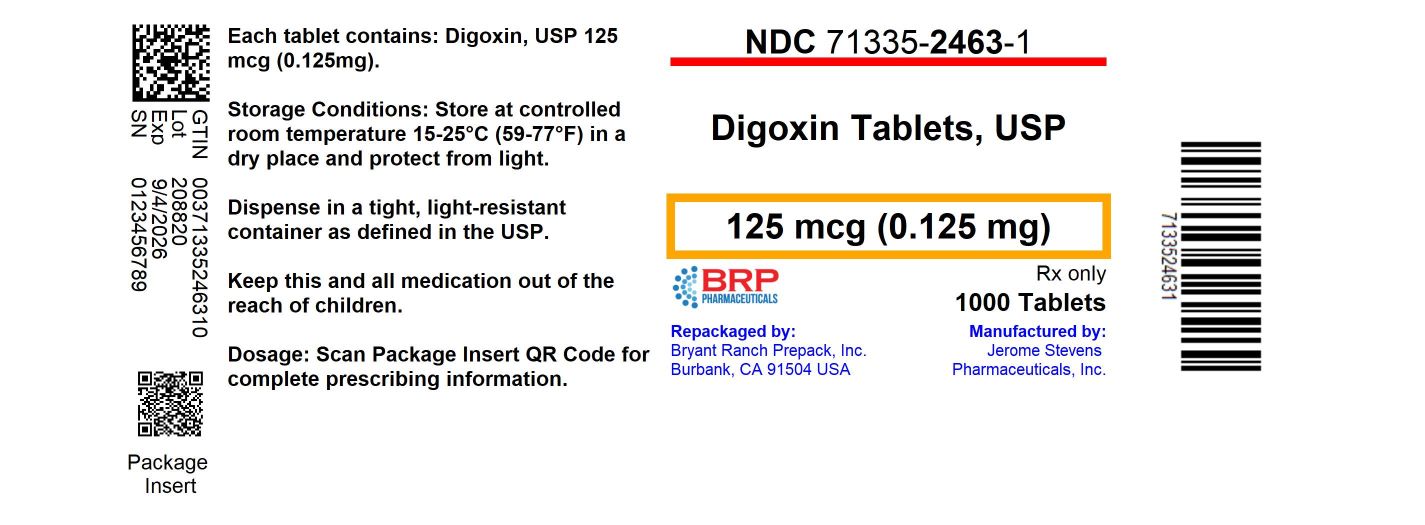
Label: DIGOXIN tablet
- NDC Code(s): 71335-2463-1, 71335-2463-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 82685-201
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Digoxin safely and effectively. See full prescribing information for Digoxin. Digoxin (Digoxin) TABLET for ORAL use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE
1.1 Heart Failure in Adults - Digoxin is indicated for the treatment of mild to moderate heart failure in adults. Digoxin increases left ventricular ejection fraction and improves heart failure ...
-
2 DOSAGE & ADMINISTRATION
Digoxin dose is based on patient-specific factors (age, lean body weight, renal function, etc.). See full prescribing information. Monitor for toxicity and therapeutic effect. 2.1 Important ...
-
3 DOSAGE FORMS & STRENGTHS
125 mcg tablets are yellow, round, scored tablets imprinted with “JSP-544”. 250 mcg tablets are white, round, scored tablets imprinted with “JSP-545”.
-
4 CONTRAINDICATIONS
Digoxin is contraindicated in patients with: Ventricular fibrillation - [see - Warnings and Precautions (5.1)] Known hypersensitivity to digoxin (reactions seen include unexplained ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Ventricular Fibrillation in Patients With Accessory AV Pathway (Wolff-Parkinson- White Syndrome) Patients with Wolff-Parkinson-White syndrome who develop atrial fibrillation are at high ...
-
6 ADVERSE REACTIONS
The following adverse reactions are included in more detail in the Warnings and Precautions section of the label: Cardiac arrhythmias - [see - Warnings and Precautions (5.1 ...
-
7 DRUG INTERACTIONS
Digoxin has a narrow therapeutic index, increased monitoring of serum digoxin concentrations and for potential signs and symptoms of clinical toxicity is necessary when initiating, adjusting, or ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Experience with digoxin in pregnant women over several decades, based on published retrospective clinical studies and case reports, has not led to the ...
-
10 OVERDOSAGE
10.1 Signs and Symptoms in Adults and Children - The signs and symptoms of toxicity are generally similar to those described in the Adverse Reactions (6.1) but may be more frequent and can be ...
-
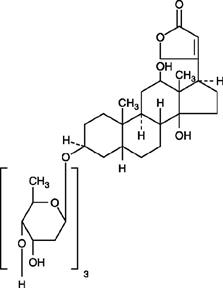
11 DESCRIPTION
Digoxin is one of the cardiac (or digitalis) glycosides, a closely related group of drugs having in common specific effects on the myocardium. These drugs are found in a number of plants. Digoxin ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - All of digoxin’s actions are mediated through its effects on Na-K ATPase. This enzyme, the “sodium pump,” is responsible for maintaining the intracellular milieu ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Digoxin showed no genotoxic potential in in vitro studies (Ames test and mouse lymphoma). No data are available on the carcinogenic ...
-
14 CLINICAL STUDIES
14.1 Chronic Heart Failure - Two 12-week, double-blind, placebo-controlled studies enrolled 178 (RADIANCE trial) and - 88 (PROVED trial) adult patients with NYHA Class II or III heart failure ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Digoxin Tablets, USP, 125 mcg (0.125 mg), Scored I.D. Imprint JSP-544 (yellow): NDC: 71335-2463-1: 1000 Tablets in a BOTTLE - NDC: 71335-2463-2: 100 Tablets in a BOTTLE - Store at controlled room ...
-
17 PATIENT COUNSELING INFORMATION
Advise patients that many drugs can interact with digoxin. Instruct patients to inform their doctor and pharmacist if they are taking any over the counter medications, including herbal ...
-
PRINCIPAL DISPLAY PANELDigoxin 125 mcg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information