Unless otherwise noted, visual acuity was measured at a distance of 4 meters.
14.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD)
The safety and efficacy of ranibizumab were assessed ...
Unless otherwise noted, visual acuity was measured at a distance of 4 meters.
14.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD)
The safety and efficacy of ranibizumab were assessed in three randomized, double-masked, sham- or active-controlled studies in patients with neovascular AMD. A total of 1323 patients (ranibizumab 879, control 444) were enrolled in the three studies.
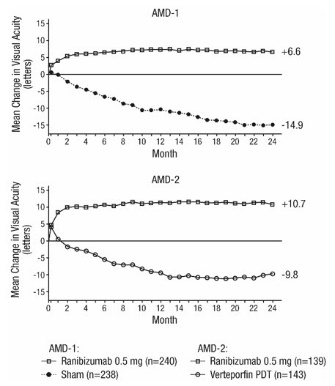
Studies AMD-1 and AMD-2
In Study AMD-1, patients with minimally classic or occult (without classic) CNV lesions received monthly ranibizumab 0.3 mg or 0.5 mg intravitreal injections or monthly sham injections. Data are available through Month 24. Patients treated with ranibizumab in Study AMD-1 received a mean of 22 total treatments out of a possible 24 from Day 0 to Month 24.
In Study AMD-2, patients with predominantly classic CNV lesions received one of the following: 1) monthly ranibizumab 0.3 mg intravitreal injections and sham PDT; 2) monthly ranibizumab 0.5 mg intravitreal injections and sham PDT; or 3) sham intravitreal injections and active PDT. Sham PDT (or active PDT) was given with the initial ranibizumab (or sham) intravitreal injection and every 3 months thereafter if FA showed persistence or recurrence of leakage. Data are available through Month 24. Patients treated with ranibizumab in Study AMD-2 received a mean of 21 total treatments out of a possible 24 from Day 0 through Month 24.
In both studies, the primary efficacy endpoint was the proportion of patients who maintained vision, defined as losing fewer than 15 letters of visual acuity at 12 months compared with baseline. Almost all ranibizumab-treated patients (approximately 95%) maintained their visual acuity. Among ranibizumab-treated patients, 31% to 37% experienced a clinically significant improvement in vision, defined as gaining 15 or more letters at 12 months. The size of the lesion did not significantly affect the results. Detailed results are shown in Table 3, Table 4, and Figure 1 below.
Patients in the group treated with ranibizumab had minimal observable CNV lesion growth, on average. At Month 12, the mean change in the total area of the CNV lesion was 0.1-0.3 disc areas (DA) for ranibizumab versus 2.3-2.6 DA for the control arms. At Month 24, the mean change in the total area of the CNV lesion was 0.3-0.4 DA for ranibizumab versus 2.9-3.1 DA for the control arms.
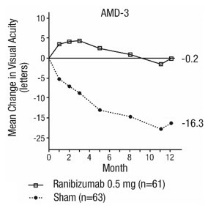
Study AMD-3
Study AMD-3 was a randomized, double-masked, sham-controlled, 2-year study designed to assess the safety and efficacy of ranibizumab in patients with neovascular AMD (with or without a classic CNV component). Data are available through Month 12. Patients received ranibizumab 0.3 mg or 0.5 mg intravitreal injections or sham injections once a month for three consecutive doses, followed by a dose administered once every 3 months for 9 months. A total of 184 patients were enrolled in this study (ranibizumab 0.3 mg, 60; ranibizumab 0.5 mg, 61; sham, 63); 171 (93%) completed 12 months of this study. Patients treated with ranibizumab in Study AMD-3 received a mean of six total treatments out of a possible 6 from Day 0 through Month 12.
In Study AMD-3, the primary efficacy endpoint was the mean change in visual acuity at 12 months compared with baseline (see Figure 2). After an initial increase in visual acuity (following monthly dosing), on average, patients dosed once every 3 months with ranibizumab lost visual acuity, returning to baseline at Month 12. In Study AMD-3, almost all ranibizumab-treated patients (90%) lost fewer than 15 letters of visual acuity at Month 12.
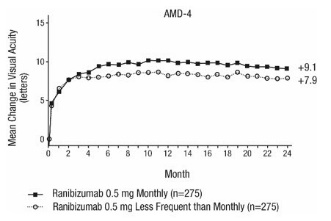
Study AMD-4
Study AMD-4 was a randomized, double-masked, active treatment-controlled, two-year study designed to assess the safety and efficacy of ranibizumab 0.5 mg administered monthly or less frequently than monthly in patients with neovascular AMD. Patients randomized to the ranibizumab 0.5 mg less frequent dosing arm received three monthly doses followed by monthly assessments where patients were eligible to receive ranibizumab injections guided by pre-specified re-treatment criteria. A total of 550 patients were enrolled in the two 0.5 mg treatment groups with 467 (85%) completing through Month 24. Data are available through Month 24.
Clinical results at Month 24 remain similar to that observed at Month 12.
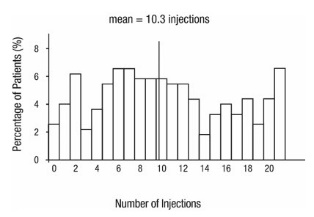
From Month 3 through Month 24, visual acuity decreased by 0.3 letters in the 0.5 mg less frequent dosing arm and increased by 0.7 letters in the 0.5 mg monthly arm (see Figure 3). Over this 21-month period, patients in the 0.5 mg less frequent dosing and the 0.5 mg monthly arms averaged 10.3 and 18.5 injections, respectively. The distribution of injections received in the less frequent dosing arm is shown in Figure 4.
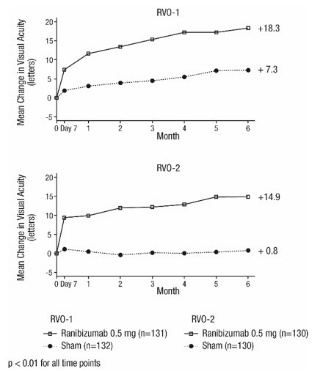
14.2 Macular Edema Following Retinal Vein Occlusion (RVO)
The safety and efficacy of ranibizumab were assessed in two randomized, double-masked, 1-year studies in patients with macular edema following RVO. Sham controlled data are available through Month 6. Patient age ranged from 20 to 91 years, with a mean age of 67 years. A total of 789 patients (ranibizumab 0.3 mg, 266 patients; ranibizumab 0.5 mg, 261 patients; sham, 262 patients) were enrolled, with 739 (94%) patients completing through Month 6. All patients completing Month 6 were eligible to receive ranibizumab injections guided by pre-specified re-treatment criteria until the end of the studies at Month 12.
In Study RVO-1, patients with macular edema following branch or hemi-RVO, received monthly ranibizumab 0.3 mg or 0.5 mg intravitreal injections or monthly sham injections for 6 months. All patients were eligible for macular focal/grid laser treatment beginning at Month 3 of the 6-month treatment period. Macular focal/grid laser treatment was given to 26 of 131 (20%) patients treated with 0.5 mg ranibizumab and 71 of 132 (54%) patients treated with sham.
In Study RVO-2, patients with macular edema following central RVO received monthly ranibizumab 0.3 mg or 0.5 mg intravitreal injections or monthly sham injections for 6 months.
At Month 6, after monthly treatment with 0.5 mg ranibizumab, the following clinical results were observed:
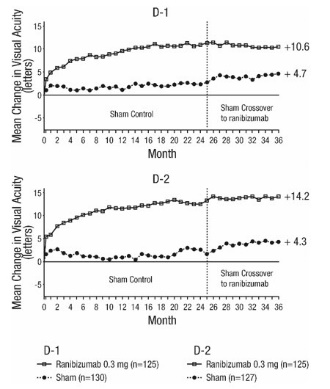
14.3 Diabetic Macular Edema (DME)
Efficacy and safety data of ranibizumab are derived from studies D-1 and D-2 [see Clinical Studies (14.4)]. All enrolled patients had DR and DME at baseline.
The safety and efficacy of ranibizumab were assessed in two randomized, double-masked, 3-year studies. The studies were sham-controlled through Month 24. Patient age ranged from 21 to 91 years, with a mean age of 62 years. A total of 759 patients (ranibizumab 0.3 mg, 250 patients; ranibizumab 0.5 mg, 252 patients; sham, 257 patients) were enrolled, with 582 (77%) completing through Month 36.
In Studies D-1 and D-2, patients received monthly ranibizumab 0.3 mg or 0.5 mg intravitreal injections or monthly sham injections during the 24-month controlled treatment period. From Months 25 through 36, patients who previously received sham were eligible to receive monthly ranibizumab 0.5 mg and patients originally randomized to monthly ranibizumab 0.3 mg or 0.5 mg continued to receive their assigned dose. All patients were eligible for macular focal/grid laser treatment beginning at Month 3 of the 24-month treatment period or panretinal photocoagulation (PRP) as needed. Through Month 24, macular focal/grid laser treatment was administered in 94 of 250 (38%) patients treated with ranibizumab 0.3 mg and 185 of 257 (72%) patients treated with sham; PRP was administered in 2 of 250 (1%) patients treated with ranibizumab 0.3 mg and 30 of 257 (12%) patients treated with sham.
Compared to monthly ranibizumab 0.3 mg, no additional benefit was observed with monthly treatment with ranibizumab 0.5 mg. At Month 24, after monthly treatment with ranibizumab 0.3 mg, the following clinical results were observed:
Visual acuity outcomes observed at Month 24 in patients treated with ranibizumab 0.3 mg were maintained with continued treatment through Month 36 in both DME studies. Patients in the sham arms who received ranibizumab 0.5 mg beginning at Month 25 achieved lesser VA gains compared to patients who began treatment with ranibizumab at the beginning of the studies.
In Studies D-1 and D-2, patients received monthly injections of ranibizumab for 12 or 36 months, after which 500 patients opted to continue in the long-term follow-up study. Of 298 patients who had at least 12 months of follow-up from Month 36, 58 (19.5%) patients maintained vision with no further therapy. The remaining 202 patients were followed for less than 12 months.
14.4 Diabetic Retinopathy (DR)
Efficacy and safety data of ranibizumab are derived from Studies D-1 and D-2 [see Clinical Studies (14.3)] and D-3. All enrolled patients in Studies D-1 and D-2 had DR and DME at baseline. Study D-3 enrolled DR patients both with and without DME at baseline.
Of the 759 patients enrolled in Studies D-1 and D-2, 746 patients had a baseline assessment of fundus photography. Patients had baseline Early Treatment Diabetic Retinopathy Study Diabetic Retinopathy Severity Scores (ETDRS-DRSS) ranging from 10 to 75. At baseline, 62% of patients had non-proliferative diabetic retinopathy (NPDR) (ETDRS-DRSS less than 60) and 31% had proliferative diabetic retinopathy (PDR) (ETDRS-DRSS greater than or equal to 60). The ETDRS-DRSS could not be graded in 5% of patients at baseline, and 2% of patients had absent or questionable DR at baseline. Approximately 20% of the overall population had prior PRP.
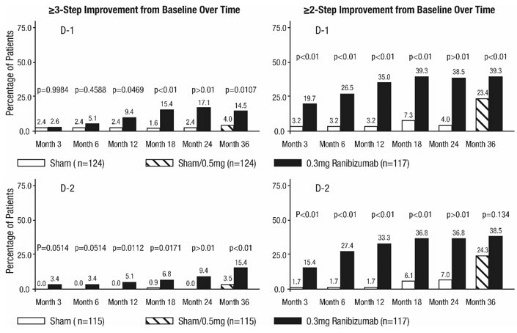
After monthly treatment with ranibizumab 0.3 mg, the following clinical results were observed (Table 7; Figure 7):
At Month 24, DR improvement by ≥3-steps in ETDRS-DRSS from baseline in subgroups examined (e.g., age, gender, race, baseline visual acuity, baseline HbA1c, prior DME therapy at baseline, baseline DR severity (NPDR, PDR)) were generally consistent with the results in the overall population.
The difference in the proportion of patients treated with ranibizumab 0.3 mg compared to sham who achieved DR improvement based on the ETDRS-DRSS was observed as early as Month 3 for ≥2-step improvement or at Month 12 for ≥3-step improvement.
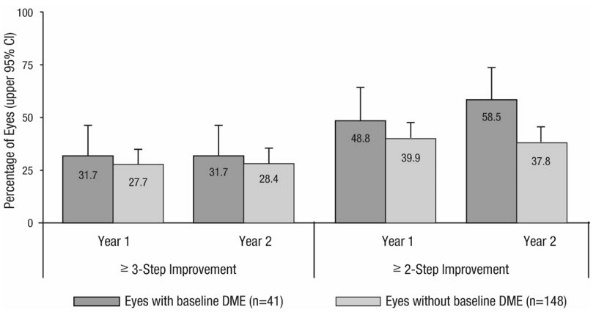
Study D-3 enrolled DR patients with and without DME; 88 (22%) eyes with baseline DME and 306 (78%) eyes without baseline DME and balanced across treatment groups. Study D-3 was a randomized, active-controlled study where patient age ranged from 20 to 83 with a mean age of 51 years. A total of 394 study eyes from 305 patients, including 89 who had both eyes randomized, were enrolled (ranibizumab, 191 study eyes; pan-retinal photocoagulation; 203 study eyes). All eyes in the ranibizumab group received a baseline 0.5 mg intravitreal injection followed by 3 monthly intravitreal injections, after which treatment was guided by pre-specified retreatment criteria. Patients had baseline ETDRS-DRSS ranging from 20 to 85. At baseline, 11% of eyes had NPDR (ETDRS-DRSS less than 60), 50% had mild-to-moderate PDR (ETDRS-DRSS equal to 60, 61, or 65), and 37% had high-risk PDR (ETDRS-DRSS greater than or equal to 71).
An analysis of data from Study D-3 demonstrated that at Year 2 in the ranibizumab group, 31.7% and 28.4% of eyes in the subgroups with baseline DME and without baseline DME, respectively, had ≥ 3-step improvement from baseline in ETDRS-DRSS.
14.5 Myopic Choroidal Neovascularization (mCNV)
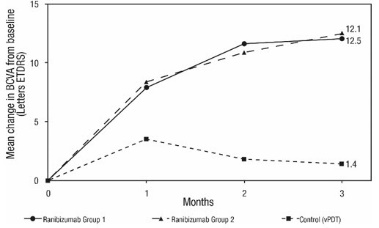
The efficacy and safety data of ranibizumab were assessed in a randomized, double-masked, active-controlled 3-month study in patients with mCNV. Patients age ranged from 18 to 87 years, with a mean age of 55 years. A total of 276 patients (222 patients in the ranibizumab treated Groups I and II; 55 patients in the active control PDT group) were enrolled. Patients randomized to the ranibizumab groups received injections guided by prespecified re-treatment criteria. The retreatment criteria in Group I were vision stability guided, with the Best Corrected Visual Acuity (BCVA) at the current visit being assessed for changes compared with the two preceding monthly BCVA values. The retreatment criteria in Group II were disease activity guided, based on BCVA decrease from the previous visit that was attributable to intra- or sub-retinal fluid or active leakage secondary to mCNV as assessed by OCT and/or FA compared to the previous monthly visit.
Visual gains for the two ranibizumab 0.5 mg treatment arms were superior to the active control arm. The mean change in BCVA from baseline at Month 3 was: +12.1 letters for Group I, +12.5 letters for Group II and +1.4 letters for the PDT group. (Figure 9; Table 9). Efficacy was comparable between Group I and Group II.
The proportion of patients who gained ≥15 letters (ETDRS) by Month 3 was 37.1% and 40.5% for ranibizumab Groups I and II, respectively and 14.5% for the PDT group. The mean number of injections between baseline and Month 3 was 2.5 and 1.8 for Groups I and II, respectively. 41% of patients received 1, 2 or 3 injections between baseline and Month 3 with no injections afterwards.
Close