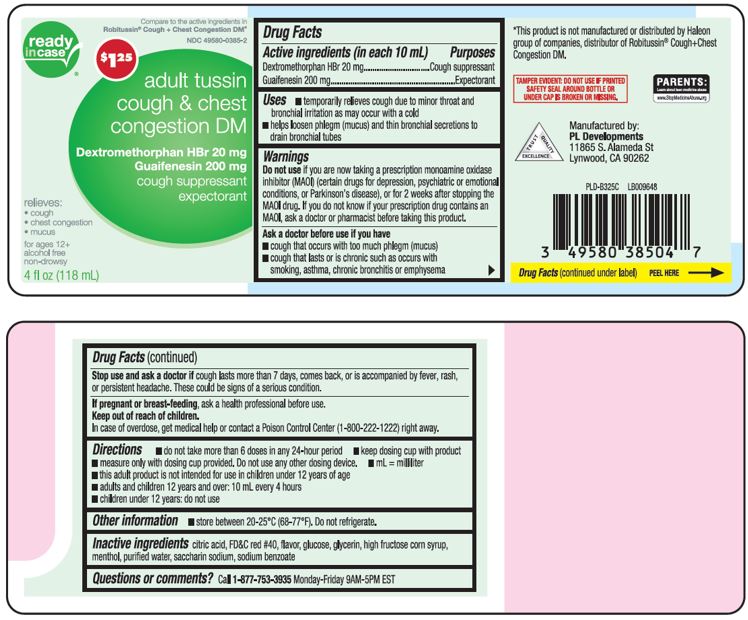
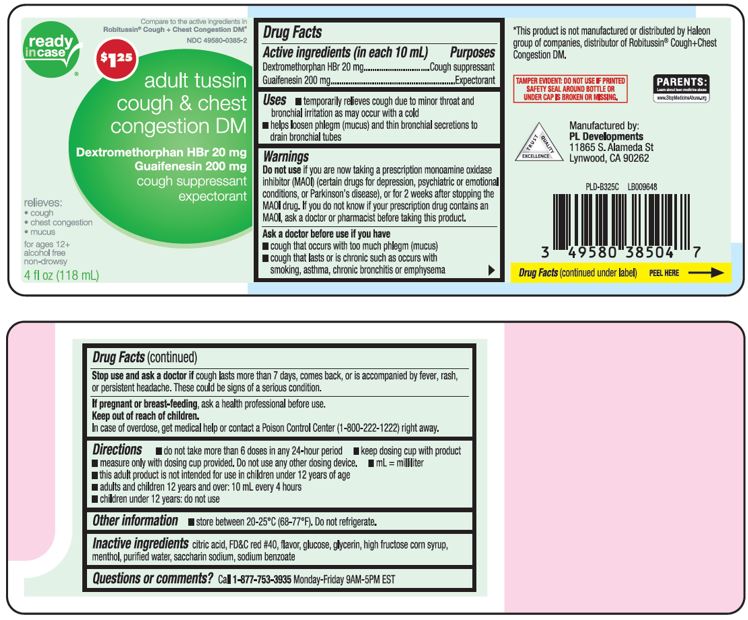
Label: TUSSIN DM COUGH AND CHEST CONGESTION ADULT- dextromethorphan hbr, guaifenesin liquid
- NDC Code(s): 49580-0385-2, 49580-0385-4, 49580-0385-8
- Packager: P & L Development, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients (in each 10 mL)Dextromethorphan HBr 20 mg - Guaifenesin 200 mg
-
PurposesCough suppressant - Expectorant
-
Usestemporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold - helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
-
WarningsDo not use - if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks ...
-
Directionsdo not take more than 6 doses in any 24-hour period - measure only with dosing cup provided. Do not use any other dosing device. keep dosing cup with product - mL= milliliter - this adult product is ...
-
Other informationstore between 20-25ºC (68-77ºF). Do not refrigerate.
-
Inactive ingredientscitric acid, FD&C red# 40, flavor, glucose, glycerin, high fructose corn syrup, menthol, purified water, saccharin sodium, sodium benzoate
-
Questions or comments?Call 1-877-753-3935 Monday-Friday 9AM-5PM EST
-
Principal Display PanelCompare to the active ingredients in Robitussin® Cough + Chest Congestion DM* adult tussin cough + chest congestion DM - Dextromethorphan HBr 20 mg - Guaifenesin 200 mg - cough ...
-
Package LabelAdult tussin Cough & Chest Congestion DM Dextromethorphan HBr 20mg; Guaifenesin 200 mg - Adult tussin Cough & Chest Congestion DM Dextromethorphan HBr 20mg; Guaifenesin 200 mg
-
INGREDIENTS AND APPEARANCEProduct Information