Label: LANSOPRAZOLE capsule, delayed release
- NDC Code(s): 43598-109-27, 43598-109-33, 43598-109-52
- Packager: Dr. Reddy's Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
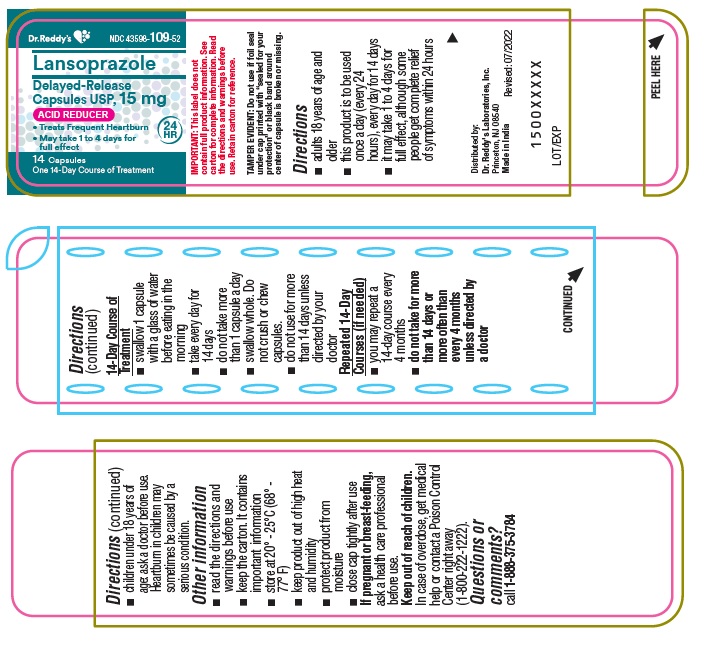
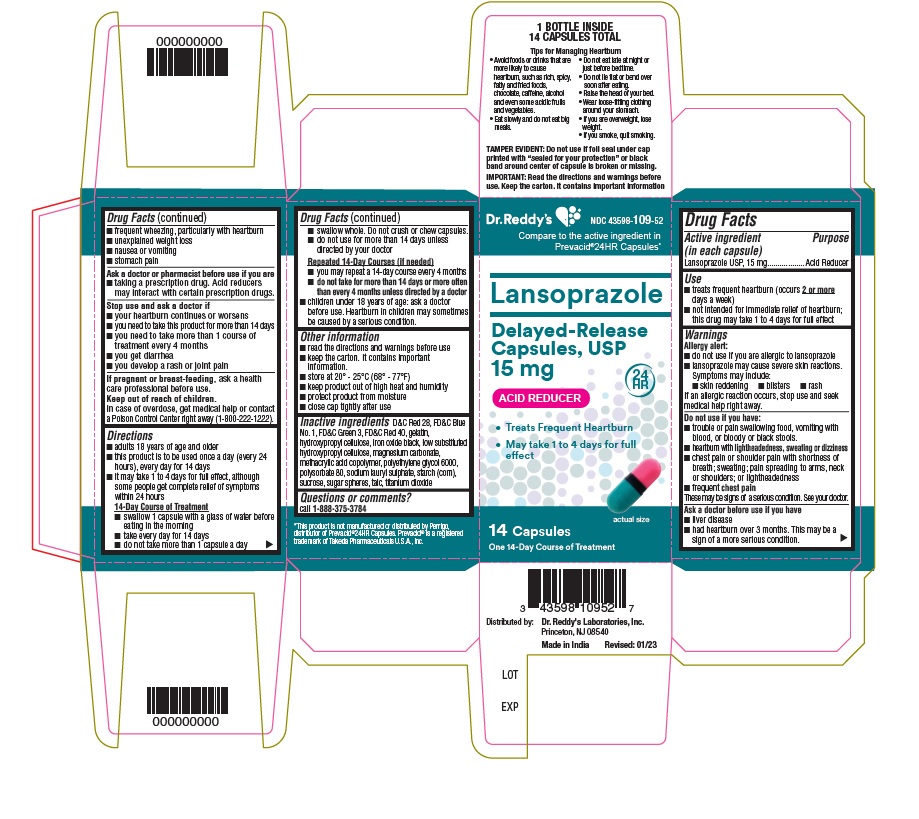
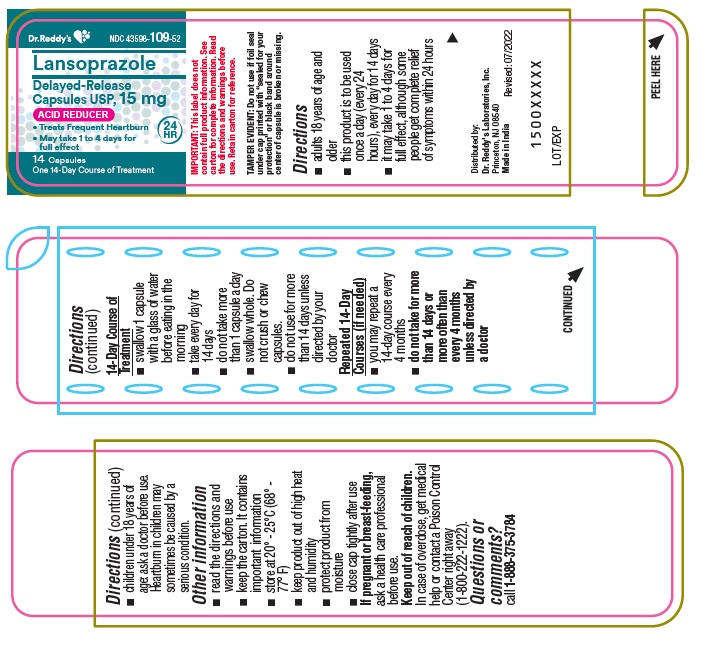
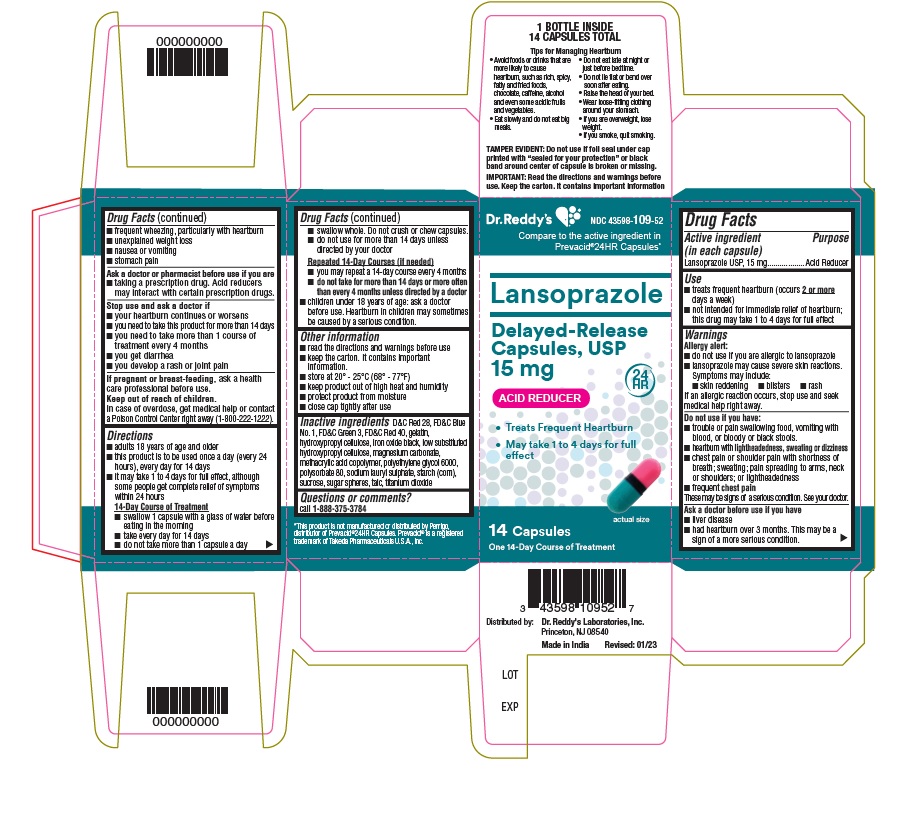
Active ingredient (in each capsule)Lansoprazole USP, 15 mg
-
PurposeAcid Reducer
-
Usetreats frequent heartburn (occurs 2 or more days a week) not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
-
WarningsAllergy alert: do not use if you are allergic to lansoprazole - lansoprazole may cause severe skin reactions. Symptoms may include: skin reddening - blisters - rash - If an allergic reaction ...
-
Directionsadults 18 years of age and older - this product is to be used once a day (every 24 hours), every day for 14 days - it may take 1 to 4 days for full effect, although some people get complete relief of ...
-
Other informationread the directions and warnings before use - keep the carton. It contains important information. store at 20 – 25°C (68° – 77° F) keep product out of high heat and humidity - protect product from ...
-
Inactive ingredientsD&C Red 28, FD&C Blue No.1, FD&C Green 3, FD&C Red 40, gelatin, hydroxypropyl cellulose, iron oxide black, low substituted hydroxypropyl cellulose, magnesium carbonate, methacrylic acid copolymer ...
-
Questions or comments?
call 1-888-375-3784
-
SPL UNCLASSIFIED SECTIONTips For Managing Heartburn - • Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits ...
-
Principal Display PanelContainer : 14's count
-
PRINCIPAL DISPLAY PANELContainer carton : 14's count
-
INGREDIENTS AND APPEARANCEProduct Information