Label: ERIBULIN MESYLATE solution
- NDC Code(s): 81298-3890-1
- Packager: Long Grove Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ERIBULIN MESYLATE INJECTION safely and effectively. See full prescribing information for ERIBULIN MESYLATE INJECTION. ERIBULIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
1.1 Metastatic Breast Cancer - Eribulin mesylate injection is indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose - The recommended dose of eribulin mesylate injection is 1.4 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. The recommended dose of ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 1 mg/2 mL (0.5 mg/mL) eribulin mesylate is a clear, colorless, sterile solution in a single-dose vial.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Neutropenia - In Study 1, severe neutropenia (ANC < 500/mm3) lasting more than one week occurred in 12% (62/503) of patients with metastatic breast cancer, leading to discontinuation in <1 ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Eribulin Mesylate Injection - No drug-drug interactions are expected with CYP3A4 inhibitors, CYP3A4 inducers or P-glycoprotein (P-gp) inhibitors. Clinically ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on findings from an animal reproduction study and its mechanism of action, eribulin mesylate injection can cause fetal harm when administered to a ...
-
10 OVERDOSAGE
Overdosage of eribulin mesylate injection has been reported at approximately 4 times the recommended dose, which resulted in Grade 3 neutropenia lasting seven days and a Grade 3 hypersensitivity ...
-
11 DESCRIPTION
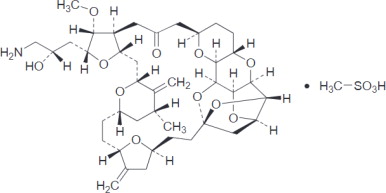
Eribulin mesylate injection contains eribulin mesylate, a microtubule dynamics inhibitor. Eribulin mesylate is a synthetic analogue of halichondrin B, a product isolated from the marine sponge ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Eribulin inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates. Eribulin exerts its ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with eribulin mesylate. Eribulin mesylate was not mutagenic in in vitro bacterial ...
-
14 CLINICAL STUDIES
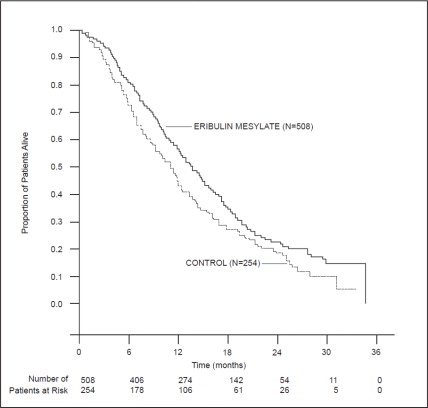
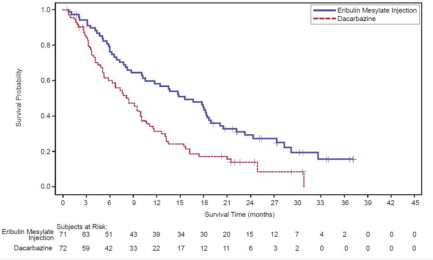
14.1 Metastatic Breast Cancer - Study 1 was an open-label, randomized, multicenter trial of 762 patients with metastatic breast cancer who received at least two chemotherapeutic regimens for the ...
-
15 REFERENCES
OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Eribulin mesylate injection is supplied as a clear colorless solution in a clear glass vial, essentially free of visible foreign particulate matter. NDC 81298-3890-1 - Injection: 1 mg/2 mL, in a ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Neutropenia - Advise patients to contact their health care provider for a fever of 100.5°F or greater or ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Eribulin Mesylate Injection - (eribu-lin me-sy-late) injection, for intravenous use - What is the most important information I should know about Eribulin Mesylate ...
-
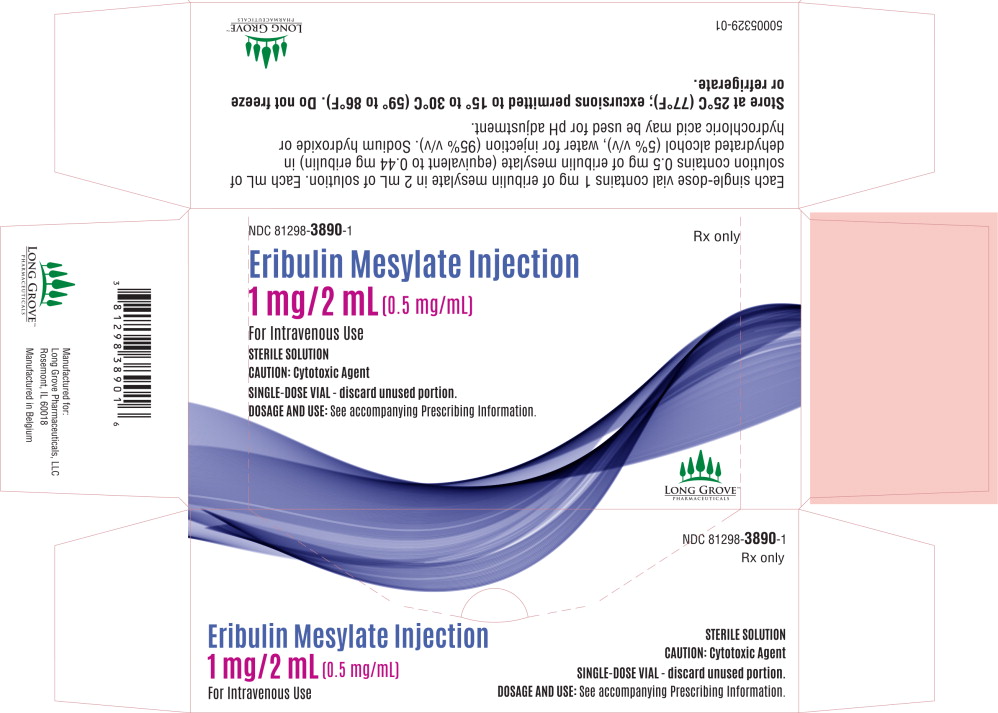
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 0.5 mg/2 mL Carton Label - NDC 81298-3890-1 - Rx only - Eribulin Mesylate Injection - 1 mg/2 mL (0.5 mg/mL) For Intravenous Use - STERILE SOLUTION - CAUTION: Cytotoxic ...
-
INGREDIENTS AND APPEARANCEProduct Information